Fold
Functional proteins very often form stable 3D structures or folds (Figure 15). However, it is generally accepted that there are a limited number of stable folds, probably on the order of thousands. The main folding unit is a structural domain.
Folds are classified into 3 main types based on their secondary structure content:
- All α, built almost exclusively from α-helixes
- All β, built almost exclusively from β-strands
- Mixed α and β, containing a mixture of both structures, either alternating α and β (α/β) or any order of α and β (α + β)
A classic example of a common mixed fold is the α/β barrel, also called the TIM barrel, consisting of eight α-helices and eight parallel β-strands that alternate along the peptide backbone. The structure is named after Triosephosphate Isomerase (TIM), a conserved glycolytic enzyme responsible for glucose degradation. This fold has been found in many different enzyme families catalysing completely unrelated reactions.
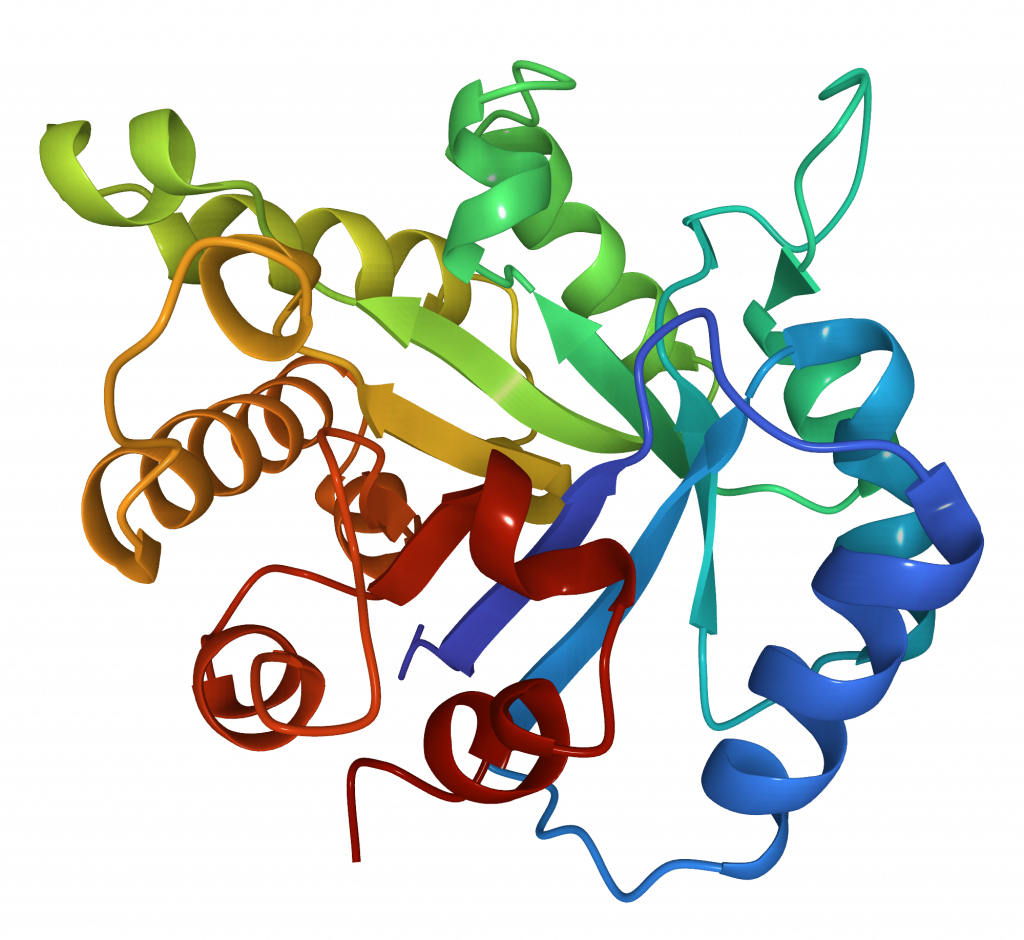
The two main resources that classify folds are: SCOP and CATH. Because of the differences in definitions and classification of folds the number of folds listed in each of them is different (7, 8, 9). Each structure in PDBe is shown using both classifications.
One of the PDBe Services called Similar Structures (PDBe Fold) can be used to compare the structures of proteins to identify conserved folds that may not be similar in sequence. An example of similar structure proteins is RsbT co-antagonist protein rsbRA (in PDBe 2bnl), which has a globin fold and shares a very similar fold structure with Sperm Whale Myoglobin (in PDBe 3o89). However, the sequence identity between these two proteins is only 12.2% (as calculated by PDBeFold).