
The protein which inspired the image for May in our 2019 calendar has become a workhorse of molecular and cell biology as well as the subject, and medium, for a lot of art.
We still don’t know why a species of jellyfish, found along the pacific coast of North America, emits green light, but we do know exactly how it does so. Aequorea victoria, the creature in question, doesn’t actually produce green light, instead it generates blue light and then modulates this to green. An enzyme called aequorin generates the blue light via the oxidation of a compound called coelenterazine, which the jellyfish obtains from its diet.
A.victoria has a second protein which absorbs the blue light produced by aequorin and emits it at the lower energy and longer wavelength green light by fluorescence. The protein responsible for this goes by the descriptive name of green fluorescent protein (GFP).
Nothing more required
Many light-absorbing proteins need to incorporate an extra molecule called a chromophore to absorb light, for example plants make chlorophyll. These molecules usually need a whole collection of enzymes to make them. When GFP was cloned in 1962, however, it was discovered that it’s chromophore was spontaneously generated within the protein. Only the GFP gene (and a bit of oxygen) was needed for a fully-functioning fluorescent protein, which makes it a perfect ‘reporter gene’. It can be added to the end of other genes and, when transcribed into protein, glows green under blue light, reporting the location of the protein it’s attached to. No other genes, enzymes, small molecules or additives are needed.
So how does GFP spontaneously make its light-absorbing-and-emitting chromophore? GFP folds up to form a barrel of beta strands, defining the environment within the protein where the chromophore sits. This environment enables a reaction between three consecutive amino acids in the core of GFP, serine, tyrosine and glycine, to form the chromophore, which absorbs blue light and emits it again in the green part of the spectrum.
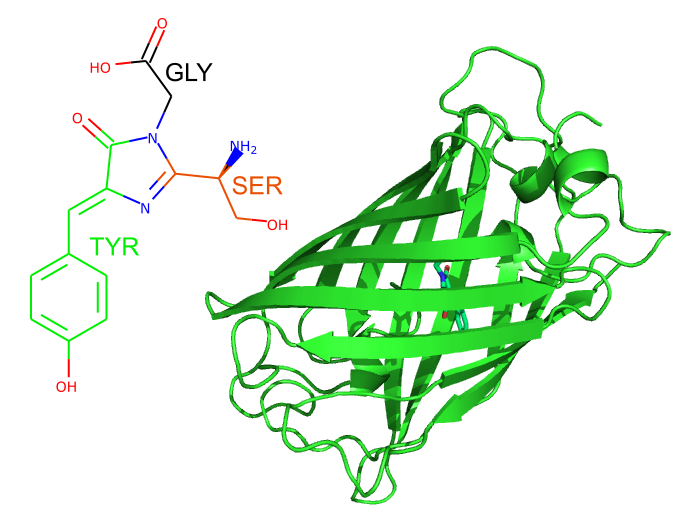
Left: The chromophore of wild-type GFP, with the three amino acids from which it is formed highlighted. Right: The overall shape of GFP, as shown in cartoons. The chromophore is just visible shown in sticks in the center of the molecule.
You can have any colour you want...
Over the decades, GFP, and its homologues from other species, have been modified by mutating amino acids in and around the chromophore to improve its properties and change its colour. Now, a whole rainbow of fluorescent proteins are available, often with exotic names, which can be used by researchers to tag different proteins and locate them within a cell. Many of these have structures available in the PDB archive.
There are versions which emit red light, for instance mStrawberry, (PDB entry 2h5p) and mCherry (PDB entry 2h5q). Moving through the spectrum there's mOrange, (PDB entry 2h5o) and yellow versions of GFP such as Citrine, (PDB entry 1huy) and Venus (PDB entry 3ako). Obviously, there are green GFPs, the wild-type protein, for instance PDB entry 1ema. At the blue end of the spectrum, tagBFP (PDB entry 3m24) and cerulean (PDB entry 2wso) are to be found. GFPs have even been engineered to emit light the infrared part of the spectrum, for instance PDB entry 5yt1.
The discovery, and subsequent development of GFP won Osamu Shimomura, Martin Chalfie and Roger Tsien the 2008 Nobel Prize in Chemistry. In what was probably not an understatement, the press release described the protein as “one of the most important tools used in contemporary bioscience”.
About the art
GFP is not only a valuable scientific tool, but is a source of artistic inspiration, as can be seen in our calendar this month. Rachel Glinsman, from The Perse School won the Rouse Award for the Creative Arts for her dress made from laser-cut luminescent material shaped like the barrel structure of GFP when shown in cartoons. In the light, it is plain white, but dim the lights and the green glow can be seen, just as it can in jellyfish.
GFP has been used to inspire art before, a sculpture of the protein can be seen at the Friday Harbour Labs in Washington state, where GFP was discovered, and it has been depicted in stained glass. GFP can also be a medium for microbial art on Petri dishes growing bacteria expressing different coloured GFP in artistic shapes. But we think Rachel’s dress is the first wearable GFP art, unless you know otherwise.
MJC


