
The September image in our 2019 calendar is inspired by a molecular system that can edit DNA and the story of a statue coming to life.
A bacterial defence
CRISPR (clustered regularly interspaced short palindromic repeats)sequences are sections of bacterial DNA found to be important in the bacterial immune response. CRISPRThese clusters of repeated DNA sequences code for CRISPR RNA (crRNA) molecules that specifically target viral DNA sequences, directing them to be chopped up. These crRNA molecules are ‘guides’ for a set of bacterial enzymes, termed CRISPR-associated, or Cas, proteins. The specific crRNA sequence binds to Cas and base-pairs with the viral DNA, so bringing the DNA into the Cas enzyme where the protein does the chopping, much like molecular scissors! This is a very handy system for bacteria, with the crRNA sequence able to evolve over many populations to recognise new viral DNA sequences, thus effectively protecting the bacteria from these attacks.
Programming the scissors
The really interesting thing about the CRISPR-Cas complexes is that changing the sequence of the RNA allows the complex to target a different section of DNA. Many CRISPR-Cas systems involved several protein subunits, but Jennifer Doudna and Emmanuelle Charpentier worked on a more simple form of these complexes, containing only the Cas9 endonuclease. They found that they could fuse two crRNAs in the complex to form a single guide RNA (sgRNA) that, along with the Cas9 protein, could cut the target DNA sequence. Thus, the CRISPR-Cas9 system could operate with only one protein, and one RNA molecule. Furthermore, by changing the sequence of the sgRNA, it could be specifically programmed to target nearly any DNA sequence.
Gene editing potential
The relative simplicity of this CRISPR-Cas9 system, along with the specific targeting of DNA sequences, makes this an ideal system for studies relating to genetic modification. It allows specific regions of DNA to be targeted, enabling removal and/or addition of DNA sequences directly into the genome. The possibilities for this technique are enormous, allowing easy modification of genomes that could help treat disease or improve food production. There are however a number of ethical concerns, particularly surrounding the possibility of editing human embryos, with much progress required to ensure the safe and ethical use of this technique.
Cas9 in the PDB
It was not until 2014 when the first structures of Cas9 and the CRISPR-Cas9 complex were solved and released in the PDB. However, Just 5 years later, there are already around 50 structures of Cas9 in the archive, most of these complexes with sgRNA. The PDB entry featured in this month’s artwork is 5x2g, the structure of Cas9 from Campylobacter jejuni (cjCas9) in complex with sgRNA and its target DNA. This particular structure revealed a triple-helix structure in the sgRNA, not observed in RNA before and not predicted from the sequence. The Cas9 protein was also found to interact with both target and non-target DNA, something not seen in other Cas9 proteins. The structure helps to reveal the diversity of the different CRISPR-Cas9 systems, highlighting how much more complex they are than first thought.
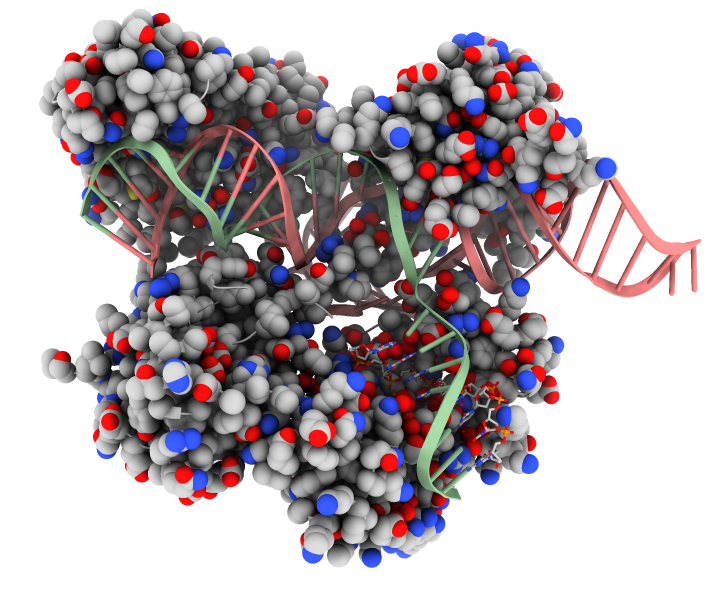
Structure of the CRISPR-Cas9 complex PDB entry 5x2g. The protein is shown as spheres coloured by element type. Nucleic acids are shown as cartoons with the target DNA in green and the sgRNA pink. Non-target DNA (bottom left) is shown as sticks
About the artwork
The artwork, entitled ‘Pygmalion 5x2g’ and created by Annabel Rogers from Stephen Perse Foundation in Cambridge, is inspired by the structure of the CRISPR-Cas9 complex and the concept of gene editing. The myth of Pygmalion, whose sculpture came to life, provides the basis to this piece, which shows the progression of a being, written in code and becoming something akin to human, The image shows the Cas9 protein and illustrates the idea of DNA being altered and changed until AI and humans become virtually indistinguishable.
David Armstrong


