
The protein which inspired the image for April in our 2020 calendar is rhodopsin, the primary photoreceptor molecule in vision. Rhodopsin is a light-sensitive G-protein coupled receptor (GPCR) containing a light-absorbing chromophore, retinal. On exposure to light, rhodopsin undergoes conformational changes, initiating a signal transduction cascade. Light is then converted into an electrical signal that is transmitted to the brain, interpreted as vision.
Opsin and retinal
The structure and function of rhodopsin has been intensely studied over the years, and rhodopsin serves as a model for understanding the GPCR family as a whole. Indeed, rhodopsin was the first GPCR to have its structure determined by x-ray crystallography.
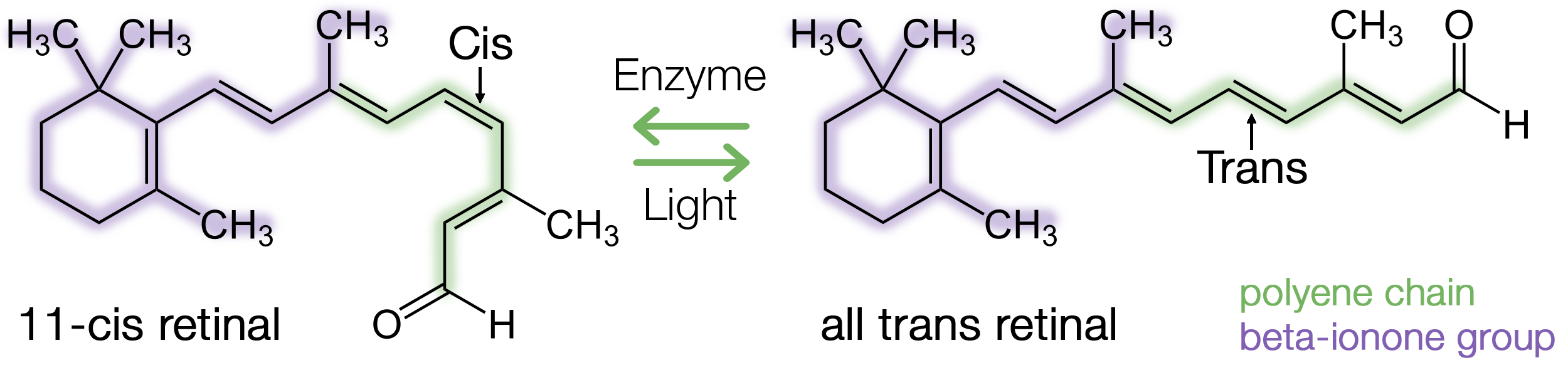
Rhodopsin is a photoreceptor composed of two parts: the opsin protein, and the 11-cis retinal chromophore which derives from vitamin A.
The retinal chromophore is composed of a polyene chain and a beta-ionone group. Retinal possesses a specific conformation because of a double bond which exists in cis between the 11th and 12th carbon in the chain.
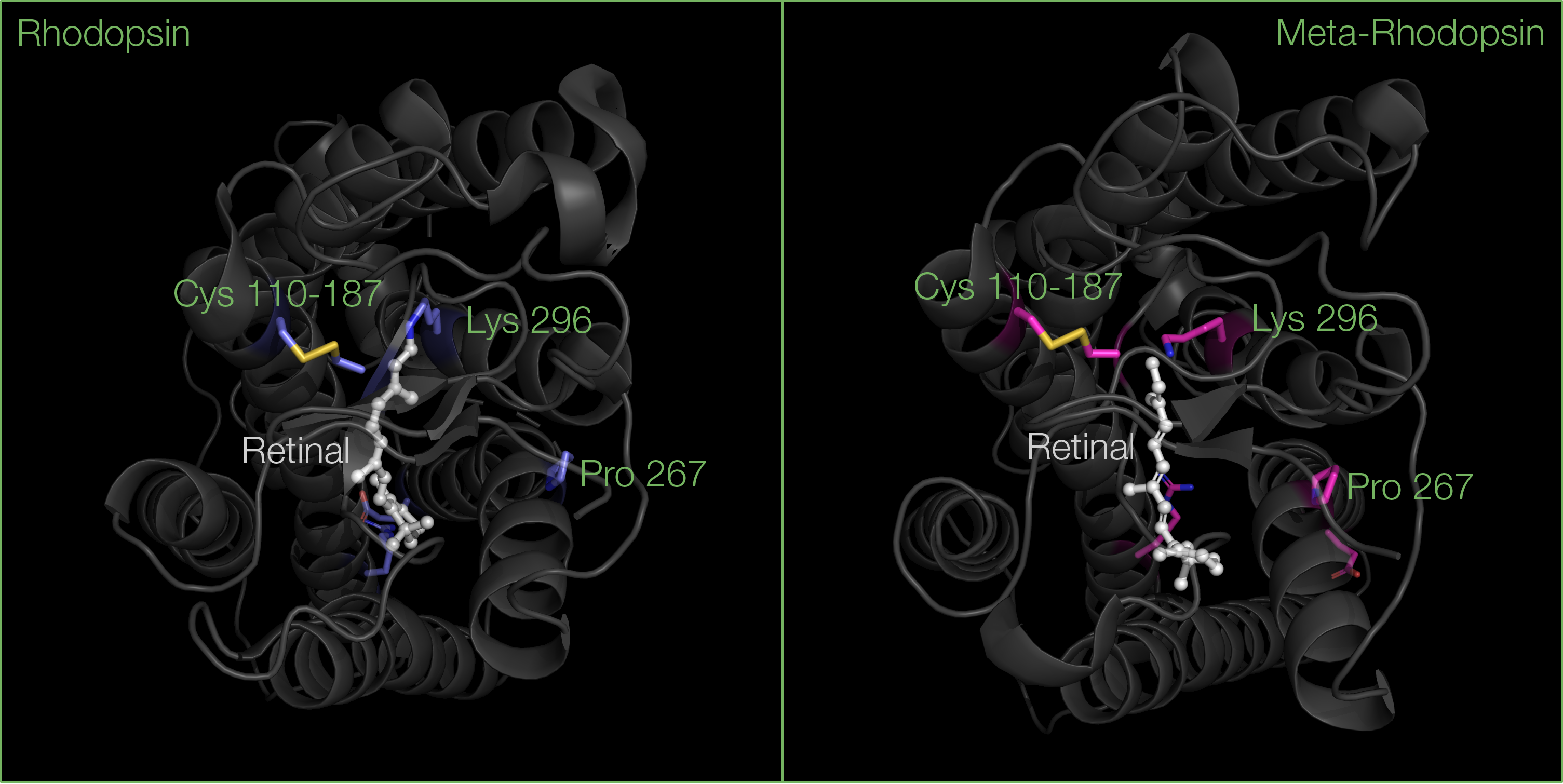
The opsin protein is composed of seven transmembrane alpha helices, as well as loops on each side of the membrane: three that extend to the extracellular matrix, and three others that extend to the cytoplasm.
The transmembrane region contains a hydrophobic core allowing the protein to sit within the non-polar membrane of rod cells. Within this hydrophobic core lies the binding pocket of retinal, where hydrophobic residues stabilize the polyene chain of retinal. The binding of retinal to opsin is also made possible through a Schiff base interaction with Lysine 296 in helix 7.
The second loop on the extracellular side of opsin is also particularly important for the binding or retinal: this region folds into two beta sheets, stabilized by a disulfide bond between conserved Cysteine residues, one located in helix 3, the other in one of the beta sheets. These beta sheets serve as a lid, blocking retinal from dissociating when rhodopsin is inactive.
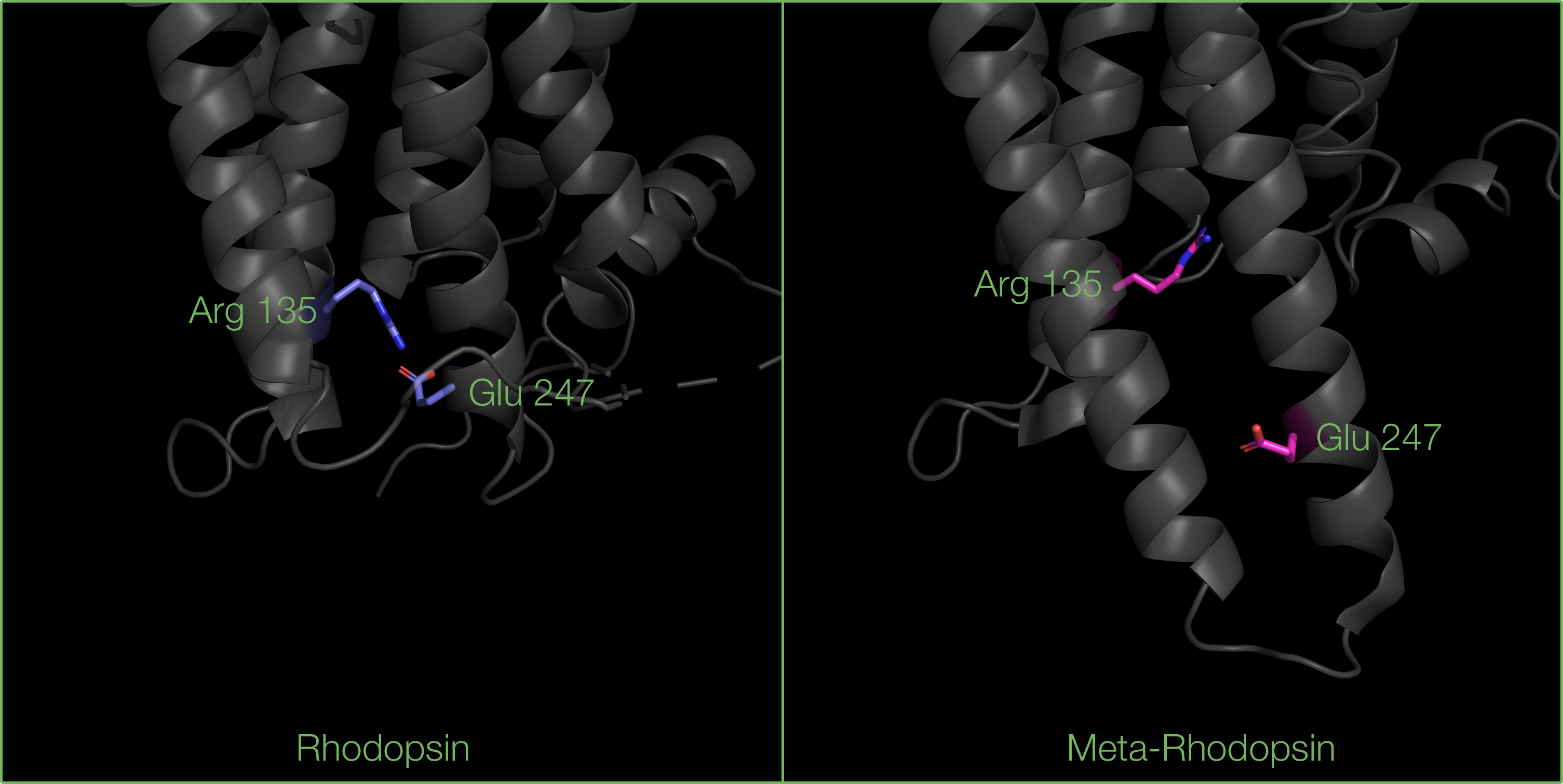
Another important feature is a salt bridge between Arginine 135 and a Glutamate 247 (located in helices 3 and 6 respectively), which prevents G-proteins from binding to inactive rhodopsin.
The photo-transduction pathway
When light is absorbed, the electrons can move, allowing the double bond between the 11th and 12th carbon in retinal to rotate, resulting in isomerization to all-trans retinal. The isomerization of retinal triggers conformational changes in opsin, and through a series of intermediates it turns into meta-rhodopsin - the active form of rhodopsin.
These conformational changes in opsin include:
- Helix 6 tilts away from the trans-membrane core towards the cytoplasmic side, due to a kink on Proline 267 residue, widening the G-protein binding site.
- Helix 5 extends into the cytoplasmic matrix, increasing the G-protein binding interface.
Breaking of the salt bridge between Arginine 135 (in helix 3) and Glutamate 247 (in helix 6) residues, revealing the G-protein binding site.
Upon photoactivation, a G-protein called transducin will bind to meta-rhodopsin. This will further activate the effector enzyme cGMP phosphodiesterase. This signalling cascade ultimately leads to a rapid visual response in rod cells.
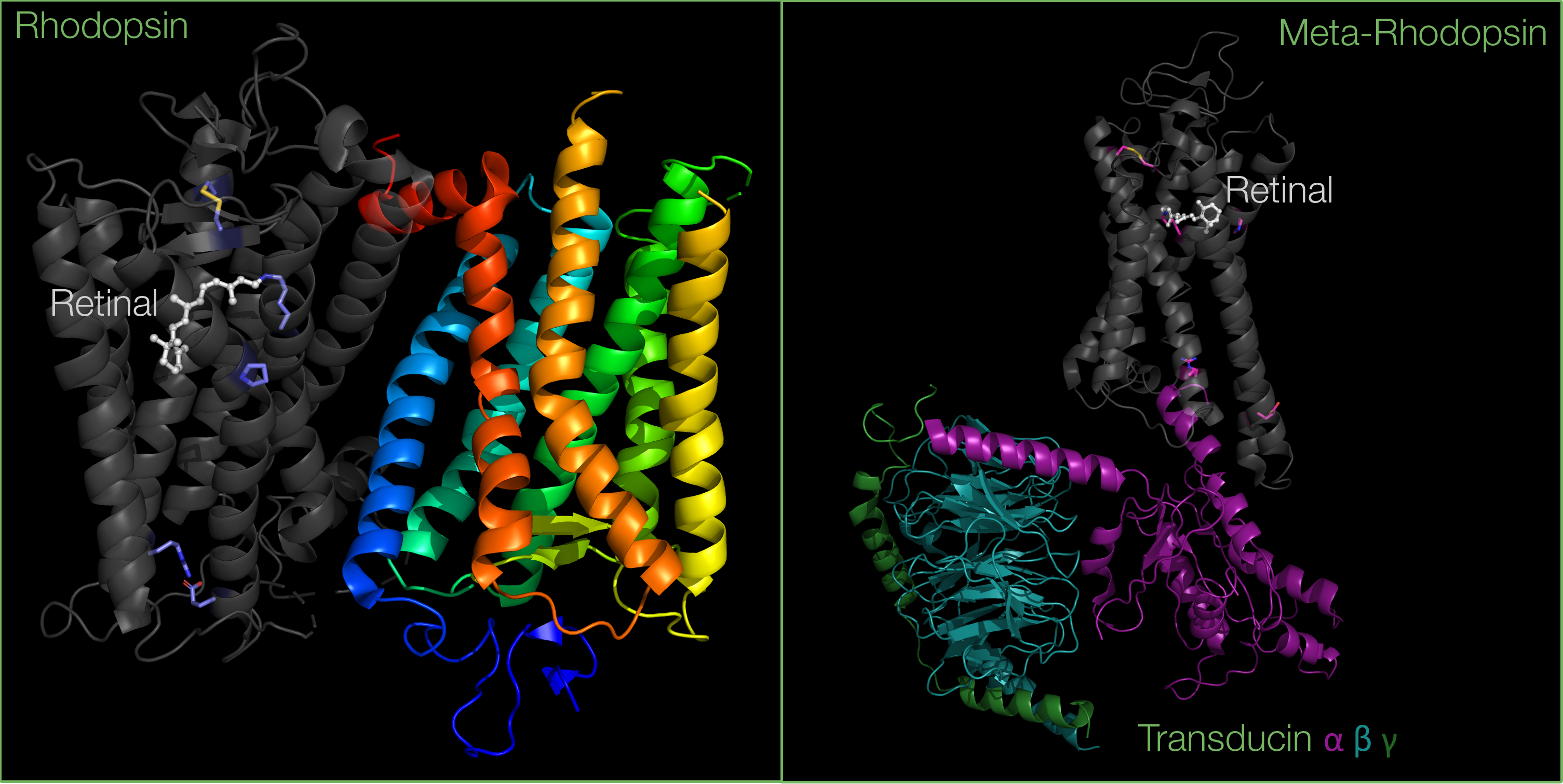
Structural and biochemical studies suggest that the rhodopsin dimer is asymmetric: it allows the interaction of transducin with the C-terminus of only one of the two rhodopsin molecules.
After the vision signal has been transmitted, meta-rhodopsin is then phosphorylated by the protein kinase GRK1, which will help recruiting another G-protein called arrestin, preventing further binding of transducin. At this point, the Schiff base linkage between all-trans retinal and Lysine 296 is hydrolysed, allowing all-trans retinal to dissociate, and releasing a phosphorylated intermediate called P-opsin. Arrestin will further dissociate, P-opsin will be dephosphorylated, becoming opsin again.
After its dissociation from P-opsin, all-trans retinal undergoes a series of enzymatic reactions before the cis-form is returned and can bind again to opsin. The key enzyme involved in the regeneration of retinal is the retinoid isomerase RPE65. The period in which retinal dissociates from opsin and needs to regenerate is known as photobleaching.
Opsin then binds 11-cis-retinal to become rhodopsin again.
Rhodopsin in blinding diseases
The photo-transduction pathway may not function properly if there are mutations in the gene encoding for the opsin protein. Defects in the rhodopsin gene are the most common cause of retinitis pigmentosa, the most common inherited blinding disease.
Several mutations have been shown to associate with structural destabilization or misfolding of opsin, leading to impaired visual function:
- the substitution of the Lysine 296 by a Methionine or Glutamate, affecting the Schiff base interaction with retinal, decreasing its binding affinity for opsin;
- mutations in the Cysteine residues involved in the stabilization of the beta sheet lid, leading to local disorder and a hindered binding of retinal;
- the substitution of Proline 267 by a Leucine or Arginine, affecting the kink in helix 6, and therefore altering the formation of the G protein binding site, limiting the binding of transducin and the subsequent signal transduction cascade;
The photo-transduction pathway involves many different photoreceptor proteins. Current challenges focus on the study of supramolecular assemblies of protein complexes responsible for visual function, as well as developing therapies to prevent blinding diseases.
About the artwork
The artwork features green and blue eyes with a superimposed protein helix representing rhodopsin and its seven helices.
Georgie de Grey, aged 17, is a student at The Leys. One of her favourite leisure pursuits is photography and also experimenting with film. She loves to visit art galleries and exhibitions to get inspired by other artists and their work.
Structures pictured in this article:
Déborah Harrus


