EMD-1226
Multiple distinct assemblies reveal conformational flexibility in the small heat shock protein Hsp26.
EMD-1226
Single-particle11.5 Å
 Deposition: 08/05/2006
Deposition: 08/05/2006Map released: 25/07/2006
Last modified: 26/05/2011
Sample Organism:
Saccharomyces cerevisiae
Sample: yeast small heat shock protein 26
Fitted models: 2h53 (Avg. Q-score: 0.061)
Deposition Authors: White HE, Orlova EV ,
Chen S,
Wang L,
Ignatiou A,
Gowen B,
Stromer T,
Franzmann TM
,
Chen S,
Wang L,
Ignatiou A,
Gowen B,
Stromer T,
Franzmann TM  ,
Haslbeck M
,
Haslbeck M  ,
Buchner J
,
Buchner J  ,
Saibil HR
,
Saibil HR
Sample: yeast small heat shock protein 26
Fitted models: 2h53 (Avg. Q-score: 0.061)
Deposition Authors: White HE, Orlova EV
 ,
Chen S,
Wang L,
Ignatiou A,
Gowen B,
Stromer T,
Franzmann TM
,
Chen S,
Wang L,
Ignatiou A,
Gowen B,
Stromer T,
Franzmann TM  ,
Haslbeck M
,
Haslbeck M  ,
Buchner J
,
Buchner J  ,
Saibil HR
,
Saibil HR
Multiple distinct assemblies reveal conformational flexibility in the small heat shock protein Hsp26.
White HE,
Orlova EV  ,
Chen S,
Wang L,
Ignatiou A,
Gowen B,
Stromer T,
Franzmann TM
,
Chen S,
Wang L,
Ignatiou A,
Gowen B,
Stromer T,
Franzmann TM  ,
Haslbeck M
,
Haslbeck M  ,
Buchner J
,
Buchner J  ,
Saibil HR
,
Saibil HR
(2006) Structure , 14 , 1197 - 1204
 ,
Chen S,
Wang L,
Ignatiou A,
Gowen B,
Stromer T,
Franzmann TM
,
Chen S,
Wang L,
Ignatiou A,
Gowen B,
Stromer T,
Franzmann TM  ,
Haslbeck M
,
Haslbeck M  ,
Buchner J
,
Buchner J  ,
Saibil HR
,
Saibil HR
(2006) Structure , 14 , 1197 - 1204
Abstract:
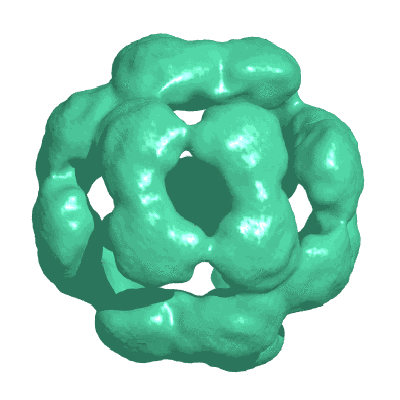
Small heat shock proteins are a superfamily of molecular chaperones that suppress protein aggregation and provide protection from cell stress. A key issue for understanding their action is to define the interactions of subunit domains in these oligomeric assemblies. Cryo-electron microscopy of yeast Hsp26 reveals two distinct forms, each comprising 24 subunits arranged in a porous shell with tetrahedral symmetry. The subunits form elongated, asymmetric dimers that assemble via trimeric contacts. Modifications of both termini cause rearrangements that yield a further four assemblies. Each subunit contains an N-terminal region, a globular middle domain, the alpha-crystallin domain, and a C-terminal tail. Twelve of the C termini form 3-fold assembly contacts which are inserted into the interior of the shell, while the other 12 C termini form contacts on the surface. Hinge points between the domains allow a variety of assembly contacts, providing the flexibility required for formation of supercomplexes with non-native proteins.
Small heat shock proteins are a superfamily of molecular chaperones that suppress protein aggregation and provide protection from cell stress. A key issue for understanding their action is to define the interactions of subunit domains in these oligomeric assemblies. Cryo-electron microscopy of yeast Hsp26 reveals two distinct forms, each comprising 24 subunits arranged in a porous shell with tetrahedral symmetry. The subunits form elongated, asymmetric dimers that assemble via trimeric contacts. Modifications of both termini cause rearrangements that yield a further four assemblies. Each subunit contains an N-terminal region, a globular middle domain, the alpha-crystallin domain, and a C-terminal tail. Twelve of the C termini form 3-fold assembly contacts which are inserted into the interior of the shell, while the other 12 C termini form contacts on the surface. Hinge points between the domains allow a variety of assembly contacts, providing the flexibility required for formation of supercomplexes with non-native proteins.
