EMD-13281
70S ribosome with EF-G, ap/P- and pe/E-site tRNAs in Mycoplasma pneumoniae cells
EMD-13281
Subtomogram averaging8.2 Å
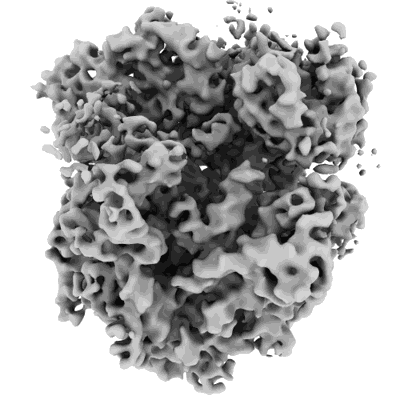 Deposition: 30/07/2021
Deposition: 30/07/2021Map released: 25/05/2022
Last modified: 06/11/2024
Sample Organism:
Mycoplasma pneumoniae M129
Sample: Cryo-electron tomograms of untreated Mycoplasma pneumoniae cells
Fitted models: 7par (Avg. Q-score: 0.174)
Deposition Authors: Xue L ,
Lenz S
,
Lenz S 
Sample: Cryo-electron tomograms of untreated Mycoplasma pneumoniae cells
Fitted models: 7par (Avg. Q-score: 0.174)
Deposition Authors: Xue L
 ,
Lenz S
,
Lenz S 
Visualizing translation dynamics at atomic detail inside a bacterial cell.
Xue L  ,
Lenz S
,
Lenz S  ,
Zimmermann-Kogadeeva M
,
Zimmermann-Kogadeeva M  ,
Tegunov D,
Cramer P
,
Tegunov D,
Cramer P  ,
Bork P
,
Bork P  ,
Rappsilber J
,
Rappsilber J  ,
Mahamid J
,
Mahamid J 
(2022) Nature , 610 , 205 - 211
 ,
Lenz S
,
Lenz S  ,
Zimmermann-Kogadeeva M
,
Zimmermann-Kogadeeva M  ,
Tegunov D,
Cramer P
,
Tegunov D,
Cramer P  ,
Bork P
,
Bork P  ,
Rappsilber J
,
Rappsilber J  ,
Mahamid J
,
Mahamid J 
(2022) Nature , 610 , 205 - 211
Abstract:
Translation is the fundamental process of protein synthesis and is catalysed by the ribosome in all living cells1. Here we use advances in cryo-electron tomography and sub-tomogram analysis2,3 to visualize the structural dynamics of translation inside the bacterium Mycoplasma pneumoniae. To interpret the functional states in detail, we first obtain a high-resolution in-cell average map of all translating ribosomes and build an atomic model for the M. pneumoniae ribosome that reveals distinct extensions of ribosomal proteins. Classification then resolves 13 ribosome states that differ in their conformation and composition. These recapitulate major states that were previously resolved in vitro, and reflect intermediates during active translation. On the basis of these states, we animate translation elongation inside native cells and show how antibiotics reshape the cellular translation landscapes. During translation elongation, ribosomes often assemble in defined three-dimensional arrangements to form polysomes4. By mapping the intracellular organization of translating ribosomes, we show that their association into polysomes involves a local coordination mechanism that is mediated by the ribosomal protein L9. We propose that an extended conformation of L9 within polysomes mitigates collisions to facilitate translation fidelity. Our work thus demonstrates the feasibility of visualizing molecular processes at atomic detail inside cells.
Translation is the fundamental process of protein synthesis and is catalysed by the ribosome in all living cells1. Here we use advances in cryo-electron tomography and sub-tomogram analysis2,3 to visualize the structural dynamics of translation inside the bacterium Mycoplasma pneumoniae. To interpret the functional states in detail, we first obtain a high-resolution in-cell average map of all translating ribosomes and build an atomic model for the M. pneumoniae ribosome that reveals distinct extensions of ribosomal proteins. Classification then resolves 13 ribosome states that differ in their conformation and composition. These recapitulate major states that were previously resolved in vitro, and reflect intermediates during active translation. On the basis of these states, we animate translation elongation inside native cells and show how antibiotics reshape the cellular translation landscapes. During translation elongation, ribosomes often assemble in defined three-dimensional arrangements to form polysomes4. By mapping the intracellular organization of translating ribosomes, we show that their association into polysomes involves a local coordination mechanism that is mediated by the ribosomal protein L9. We propose that an extended conformation of L9 within polysomes mitigates collisions to facilitate translation fidelity. Our work thus demonstrates the feasibility of visualizing molecular processes at atomic detail inside cells.
