EMD-14797
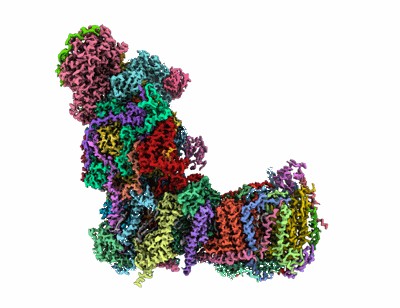
CryoEM structure of mitochondrial complex I from Chaetomium thermophilum (state 1)
EMD-14797
Single-particle2.44 Å
 Deposition: 19/04/2022
Deposition: 19/04/2022Map released: 30/11/2022
Last modified: 23/10/2024
Sample Organism:
Chaetomium thermophilum var. thermophilum DSM 1495
Sample: Mitochondrial NADH:ubiquinone oxidoreductase in LMNG
Fitted models: 7zmg (Avg. Q-score: 0.667)
Deposition Authors: Laube E ,
Kuehlbrandt W
,
Kuehlbrandt W
Sample: Mitochondrial NADH:ubiquinone oxidoreductase in LMNG
Fitted models: 7zmg (Avg. Q-score: 0.667)
Deposition Authors: Laube E
 ,
Kuehlbrandt W
,
Kuehlbrandt W
Conformational changes in mitochondrial complex I of the thermophilic eukaryote Chaetomium thermophilum.
Abstract:
Mitochondrial complex I is a redox-driven proton pump that generates proton-motive force across the inner mitochondrial membrane, powering oxidative phosphorylation and ATP synthesis in eukaryotes. We report the structure of complex I from the thermophilic fungus Chaetomium thermophilum, determined by cryoEM up to 2.4-Å resolution. We show that the complex undergoes a transition between two conformations, which we refer to as state 1 and state 2. The conformational switch is manifest in a twisting movement of the peripheral arm relative to the membrane arm, but most notably in substantial rearrangements of the Q-binding cavity and the E-channel, resulting in a continuous aqueous passage from the E-channel to subunit ND5 at the far end of the membrane arm. The conformational changes in the complex interior resemble those reported for mammalian complex I, suggesting a highly conserved, universal mechanism of coupling electron transport to proton pumping.
Mitochondrial complex I is a redox-driven proton pump that generates proton-motive force across the inner mitochondrial membrane, powering oxidative phosphorylation and ATP synthesis in eukaryotes. We report the structure of complex I from the thermophilic fungus Chaetomium thermophilum, determined by cryoEM up to 2.4-Å resolution. We show that the complex undergoes a transition between two conformations, which we refer to as state 1 and state 2. The conformational switch is manifest in a twisting movement of the peripheral arm relative to the membrane arm, but most notably in substantial rearrangements of the Q-binding cavity and the E-channel, resulting in a continuous aqueous passage from the E-channel to subunit ND5 at the far end of the membrane arm. The conformational changes in the complex interior resemble those reported for mammalian complex I, suggesting a highly conserved, universal mechanism of coupling electron transport to proton pumping.
