EMD-15681
Human leptin in complex with the human LEP-R ectodomain fused to a C-terminal trimeric isoleucine GCN4 zipper (closed 3:3 model)
EMD-15681
Single-particle6.45 Å
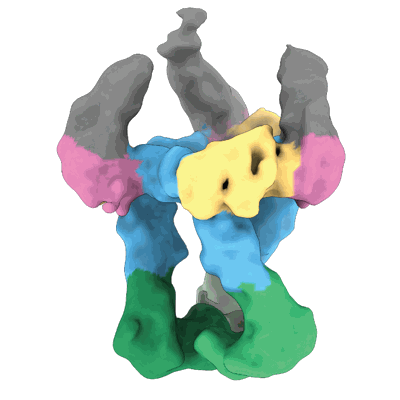 Deposition: 26/08/2022
Deposition: 26/08/2022Map released: 05/04/2023
Last modified: 20/11/2024
Sample Organism:
Homo sapiens
Sample: Human leptin in complex with the human LEP-R ectodomain C-terminally fused to a trimeric GCN4 isoleucine zipper tag
Fitted models: 8avf (Avg. Q-score: 0.098)
Deposition Authors: Verstraete K ,
Savvides SN
,
Savvides SN  ,
Verschueren KG,
Tsirigotaki A
,
Verschueren KG,
Tsirigotaki A
Sample: Human leptin in complex with the human LEP-R ectodomain C-terminally fused to a trimeric GCN4 isoleucine zipper tag
Fitted models: 8avf (Avg. Q-score: 0.098)
Deposition Authors: Verstraete K
 ,
Savvides SN
,
Savvides SN  ,
Verschueren KG,
Tsirigotaki A
,
Verschueren KG,
Tsirigotaki A
Mechanism of receptor assembly via the pleiotropic adipokine Leptin.
Tsirigotaki A,
Dansercoer A,
Verschueren KHG,
Markovic I  ,
Pollmann C
,
Pollmann C  ,
Hafer M,
Felix J
,
Hafer M,
Felix J  ,
Birck C
,
Birck C  ,
Van Putte W
,
Van Putte W  ,
Catteeuw D,
Tavernier J
,
Catteeuw D,
Tavernier J  ,
Fernando Bazan J,
Piehler J
,
Fernando Bazan J,
Piehler J  ,
Savvides SN
,
Savvides SN  ,
Verstraete K
,
Verstraete K 
(2023) Nat Struct Mol Biol , 30 , 551 - 563
 ,
Pollmann C
,
Pollmann C  ,
Hafer M,
Felix J
,
Hafer M,
Felix J  ,
Birck C
,
Birck C  ,
Van Putte W
,
Van Putte W  ,
Catteeuw D,
Tavernier J
,
Catteeuw D,
Tavernier J  ,
Fernando Bazan J,
Piehler J
,
Fernando Bazan J,
Piehler J  ,
Savvides SN
,
Savvides SN  ,
Verstraete K
,
Verstraete K 
(2023) Nat Struct Mol Biol , 30 , 551 - 563
Abstract:
The adipokine Leptin activates its receptor LEP-R in the hypothalamus to regulate body weight and exerts additional pleiotropic functions in immunity, fertility and cancer. However, the structure and mechanism of Leptin-mediated LEP-R assemblies has remained unclear. Intriguingly, the signaling-competent isoform of LEP-R is only lowly abundant amid several inactive short LEP-R isoforms contributing to a mechanistic conundrum. Here we show by X-ray crystallography and cryo-EM that, in contrast to long-standing paradigms, Leptin induces type I cytokine receptor assemblies featuring 3:3 stoichiometry and demonstrate such Leptin-induced trimerization of LEP-R on living cells via single-molecule microscopy. In mediating these assemblies, Leptin undergoes drastic restructuring that activates its site III for binding to the Ig domain of an adjacent LEP-R. These interactions are abolished by mutations linked to obesity. Collectively, our study provides the structural and mechanistic framework for how evolutionarily conserved Leptin:LEP-R assemblies with 3:3 stoichiometry can engage distinct LEP-R isoforms to achieve signaling.
The adipokine Leptin activates its receptor LEP-R in the hypothalamus to regulate body weight and exerts additional pleiotropic functions in immunity, fertility and cancer. However, the structure and mechanism of Leptin-mediated LEP-R assemblies has remained unclear. Intriguingly, the signaling-competent isoform of LEP-R is only lowly abundant amid several inactive short LEP-R isoforms contributing to a mechanistic conundrum. Here we show by X-ray crystallography and cryo-EM that, in contrast to long-standing paradigms, Leptin induces type I cytokine receptor assemblies featuring 3:3 stoichiometry and demonstrate such Leptin-induced trimerization of LEP-R on living cells via single-molecule microscopy. In mediating these assemblies, Leptin undergoes drastic restructuring that activates its site III for binding to the Ig domain of an adjacent LEP-R. These interactions are abolished by mutations linked to obesity. Collectively, our study provides the structural and mechanistic framework for how evolutionarily conserved Leptin:LEP-R assemblies with 3:3 stoichiometry can engage distinct LEP-R isoforms to achieve signaling.
