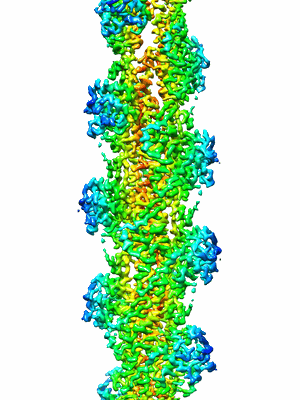
EMD-20694
Actin phalloidin at BeFx state
EMD-20694
Helical reconstruction3.8 Å
 Deposition: 06/09/2019
Deposition: 06/09/2019Map released: 13/05/2020
Last modified: 20/05/2020
Sample Organism:
Rabbit,
synthetic construct
Sample: Actin phalloidin, BeFx state
Fitted models: 6u96 (Avg. Q-score: 0.497)
Deposition Authors: Das S, Ge P, Durer ZAO, Grintsevich EE, Zhou ZH, Reisler E
Sample: Actin phalloidin, BeFx state
Fitted models: 6u96 (Avg. Q-score: 0.497)
Deposition Authors: Das S, Ge P, Durer ZAO, Grintsevich EE, Zhou ZH, Reisler E
D-loop Dynamics and Near-Atomic-Resolution Cryo-EM Structure of Phalloidin-Bound F-Actin.
Abstract:
Detailed molecular information on G-actin assembly into filaments (F-actin), and their structure, dynamics, and interactions, is essential for understanding their cellular functions. Previous studies indicate that a flexible DNase I binding loop (D-loop, residues 40-50) plays a major role in actin's conformational dynamics. Phalloidin, a "gold standard" for actin filament staining, stabilizes them and affects the D-loop. Using disulfide crosslinking in yeast actin D-loop mutant Q41C/V45C, light-scattering measurements, and cryoelectron microscopy reconstructions, we probed the constraints of D-loop dynamics and its contribution to F-actin formation/stability. Our data support a model of residues 41-45 distances that facilitate G- to F-actin transition. We report also a 3.3-Å resolution structure of phalloidin-bound F-actin in the ADP-Pi-like (ADP-BeFx) state. This shows the phalloidin-binding site on F-actin and how the relative movement between its two protofilaments is restricted by it. Together, our results provide molecular details of F-actin structure and D-loop dynamics.
Detailed molecular information on G-actin assembly into filaments (F-actin), and their structure, dynamics, and interactions, is essential for understanding their cellular functions. Previous studies indicate that a flexible DNase I binding loop (D-loop, residues 40-50) plays a major role in actin's conformational dynamics. Phalloidin, a "gold standard" for actin filament staining, stabilizes them and affects the D-loop. Using disulfide crosslinking in yeast actin D-loop mutant Q41C/V45C, light-scattering measurements, and cryoelectron microscopy reconstructions, we probed the constraints of D-loop dynamics and its contribution to F-actin formation/stability. Our data support a model of residues 41-45 distances that facilitate G- to F-actin transition. We report also a 3.3-Å resolution structure of phalloidin-bound F-actin in the ADP-Pi-like (ADP-BeFx) state. This shows the phalloidin-binding site on F-actin and how the relative movement between its two protofilaments is restricted by it. Together, our results provide molecular details of F-actin structure and D-loop dynamics.
