EMD-22679
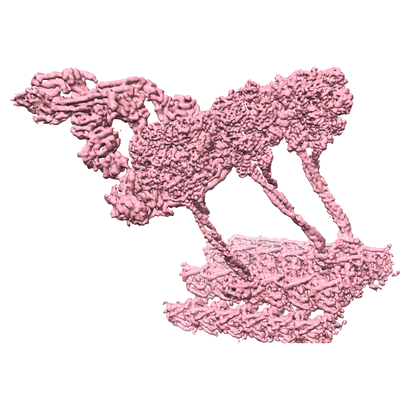
Structure of outer-arm dynein bound to microtubule doublet in microtubule binding state 2 (MTBS-2)
EMD-22679
Single-particle4.5 Å
 Deposition: 16/09/2020
Deposition: 16/09/2020Map released: 29/09/2021
Last modified: 20/11/2024
Sample Organism:
Tetrahymena thermophila
Sample: Outer-arm dynein bound to microtubule doublet in microtubule binding state 2 (MTBS-2)
Fitted models: 7k5b (Avg. Q-score: 0.264)
Deposition Authors: Rao Q ,
Zhang K
,
Zhang K 
Sample: Outer-arm dynein bound to microtubule doublet in microtubule binding state 2 (MTBS-2)
Fitted models: 7k5b (Avg. Q-score: 0.264)
Deposition Authors: Rao Q
 ,
Zhang K
,
Zhang K 
Structures of outer-arm dynein array on microtubule doublet reveal a motor coordination mechanism.
Rao Q  ,
Han L
,
Han L  ,
Wang Y
,
Wang Y  ,
Chai P
,
Chai P  ,
Kuo YW
,
Kuo YW  ,
Yang R,
Hu F,
Yang Y,
Howard J,
Zhang K
,
Yang R,
Hu F,
Yang Y,
Howard J,
Zhang K 
(2021) Nat Struct Mol Biol , 28 , 799 - 810
 ,
Han L
,
Han L  ,
Wang Y
,
Wang Y  ,
Chai P
,
Chai P  ,
Kuo YW
,
Kuo YW  ,
Yang R,
Hu F,
Yang Y,
Howard J,
Zhang K
,
Yang R,
Hu F,
Yang Y,
Howard J,
Zhang K 
(2021) Nat Struct Mol Biol , 28 , 799 - 810
Abstract:
Thousands of outer-arm dyneins (OADs) are arrayed in the axoneme to drive a rhythmic ciliary beat. Coordination among multiple OADs is essential for generating mechanical forces to bend microtubule doublets (MTDs). Using electron microscopy, we determined high-resolution structures of Tetrahymena thermophila OAD arrays bound to MTDs in two different states. OAD preferentially binds to MTD protofilaments with a pattern resembling the native tracks for its distinct microtubule-binding domains. Upon MTD binding, free OADs are induced to adopt a stable parallel conformation, primed for array formation. Extensive tail-to-head (TTH) interactions between OADs are observed, which need to be broken for ATP turnover by the dynein motor. We propose that OADs in an array sequentially hydrolyze ATP to slide the MTDs. ATP hydrolysis in turn relaxes the TTH interfaces to effect free nucleotide cycles of downstream OADs. These findings lead to a model explaining how conformational changes in the axoneme produce coordinated action of dyneins.
Thousands of outer-arm dyneins (OADs) are arrayed in the axoneme to drive a rhythmic ciliary beat. Coordination among multiple OADs is essential for generating mechanical forces to bend microtubule doublets (MTDs). Using electron microscopy, we determined high-resolution structures of Tetrahymena thermophila OAD arrays bound to MTDs in two different states. OAD preferentially binds to MTD protofilaments with a pattern resembling the native tracks for its distinct microtubule-binding domains. Upon MTD binding, free OADs are induced to adopt a stable parallel conformation, primed for array formation. Extensive tail-to-head (TTH) interactions between OADs are observed, which need to be broken for ATP turnover by the dynein motor. We propose that OADs in an array sequentially hydrolyze ATP to slide the MTDs. ATP hydrolysis in turn relaxes the TTH interfaces to effect free nucleotide cycles of downstream OADs. These findings lead to a model explaining how conformational changes in the axoneme produce coordinated action of dyneins.
