EMD-23817
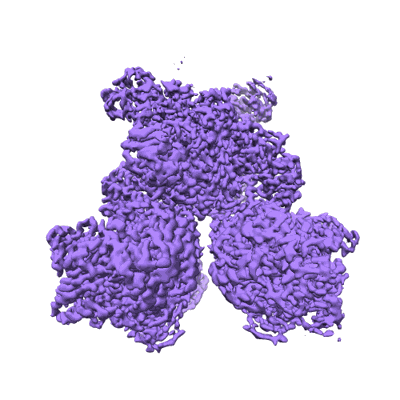
Glutamate synthase, glutamate dehydrogenase counter-enzyme complex
EMD-23817
Single-particle2.42 Å
 Deposition: 10/04/2021
Deposition: 10/04/2021Map released: 05/01/2022
Last modified: 25/12/2024
Sample Organism:
Bacillus subtilis
Sample: GudB6-GltA2-GltB2
Fitted models: 7mfm (Avg. Q-score: 0.558)
Deposition Authors: Jayaraman V ,
Lee DJ
,
Lee DJ
Sample: GudB6-GltA2-GltB2
Fitted models: 7mfm (Avg. Q-score: 0.558)
Deposition Authors: Jayaraman V
 ,
Lee DJ
,
Lee DJ
A counter-enzyme complex regulates glutamate metabolism in Bacillus subtilis.
Jayaraman V  ,
Lee DJ,
Elad N,
Vimer S
,
Lee DJ,
Elad N,
Vimer S  ,
Sharon M
,
Sharon M  ,
Fraser JS
,
Fraser JS  ,
Tawfik DS
,
Tawfik DS
(2022) Nat Chem Biol , 18 , 161 - 170
 ,
Lee DJ,
Elad N,
Vimer S
,
Lee DJ,
Elad N,
Vimer S  ,
Sharon M
,
Sharon M  ,
Fraser JS
,
Fraser JS  ,
Tawfik DS
,
Tawfik DS
(2022) Nat Chem Biol , 18 , 161 - 170
Abstract:
Multi-enzyme assemblies composed of metabolic enzymes catalyzing sequential reactions are being increasingly studied. Here, we report the discovery of a 1.6 megadalton multi-enzyme complex from Bacillus subtilis composed of two enzymes catalyzing opposite ('counter-enzymes') rather than sequential reactions: glutamate synthase (GltAB) and glutamate dehydrogenase (GudB), which make and break glutamate, respectively. In vivo and in vitro studies show that the primary role of complex formation is to inhibit the activity of GudB. Using cryo-electron microscopy, we elucidated the structure of the complex and the molecular basis of inhibition of GudB by GltAB. The complex exhibits unusual oscillatory progress curves and is necessary for both planktonic growth, in glutamate-limiting conditions, and for biofilm growth, in glutamate-rich media. The regulation of a key metabolic enzyme by complexing with its counter enzyme may thus enable cell growth under fluctuating glutamate concentrations.
Multi-enzyme assemblies composed of metabolic enzymes catalyzing sequential reactions are being increasingly studied. Here, we report the discovery of a 1.6 megadalton multi-enzyme complex from Bacillus subtilis composed of two enzymes catalyzing opposite ('counter-enzymes') rather than sequential reactions: glutamate synthase (GltAB) and glutamate dehydrogenase (GudB), which make and break glutamate, respectively. In vivo and in vitro studies show that the primary role of complex formation is to inhibit the activity of GudB. Using cryo-electron microscopy, we elucidated the structure of the complex and the molecular basis of inhibition of GudB by GltAB. The complex exhibits unusual oscillatory progress curves and is necessary for both planktonic growth, in glutamate-limiting conditions, and for biofilm growth, in glutamate-rich media. The regulation of a key metabolic enzyme by complexing with its counter enzyme may thus enable cell growth under fluctuating glutamate concentrations.
