EMD-24663
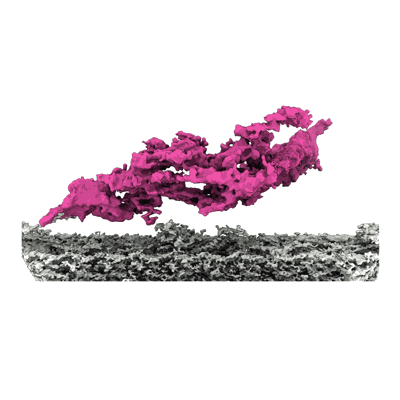
Cryo-EM density map of the outer dynein arm core from bovine tracheal cilia
EMD-24663
Single-particle8.0 Å
 Deposition: 10/08/2021
Deposition: 10/08/2021Map released: 27/10/2021
Last modified: 24/11/2021
Sample Organism:
Bos taurus
Sample: Outer dynein arm bound to doublet microtubule
Deposition Authors: Gui M ,
Anderson JR
,
Anderson JR  ,
Botsch JJ
,
Botsch JJ  ,
Meleppattu S
,
Meleppattu S  ,
Singh SK
,
Singh SK  ,
Zhang Q,
Brown A
,
Zhang Q,
Brown A
Sample: Outer dynein arm bound to doublet microtubule
Deposition Authors: Gui M
 ,
Anderson JR
,
Anderson JR  ,
Botsch JJ
,
Botsch JJ  ,
Meleppattu S
,
Meleppattu S  ,
Singh SK
,
Singh SK  ,
Zhang Q,
Brown A
,
Zhang Q,
Brown A
De novo identification of mammalian ciliary motility proteins using cryo-EM.
Gui M  ,
Farley H
,
Farley H  ,
Anujan P,
Anderson JR
,
Anujan P,
Anderson JR  ,
Maxwell DW
,
Maxwell DW  ,
Whitchurch JB,
Botsch JJ
,
Whitchurch JB,
Botsch JJ  ,
Qiu T
,
Qiu T  ,
Meleppattu S
,
Meleppattu S  ,
Singh SK
,
Singh SK  ,
Zhang Q,
Thompson J
,
Zhang Q,
Thompson J  ,
Lucas JS
,
Lucas JS  ,
Bingle CD
,
Bingle CD  ,
Norris DP,
Roy S,
Brown A
,
Norris DP,
Roy S,
Brown A
(2021) Cell , 184 , 5791 - 5806.e19
 ,
Farley H
,
Farley H  ,
Anujan P,
Anderson JR
,
Anujan P,
Anderson JR  ,
Maxwell DW
,
Maxwell DW  ,
Whitchurch JB,
Botsch JJ
,
Whitchurch JB,
Botsch JJ  ,
Qiu T
,
Qiu T  ,
Meleppattu S
,
Meleppattu S  ,
Singh SK
,
Singh SK  ,
Zhang Q,
Thompson J
,
Zhang Q,
Thompson J  ,
Lucas JS
,
Lucas JS  ,
Bingle CD
,
Bingle CD  ,
Norris DP,
Roy S,
Brown A
,
Norris DP,
Roy S,
Brown A
(2021) Cell , 184 , 5791 - 5806.e19
Abstract:
Dynein-decorated doublet microtubules (DMTs) are critical components of the oscillatory molecular machine of cilia, the axoneme, and have luminal surfaces patterned periodically by microtubule inner proteins (MIPs). Here we present an atomic model of the 48-nm repeat of a mammalian DMT, derived from a cryoelectron microscopy (cryo-EM) map of the complex isolated from bovine respiratory cilia. The structure uncovers principles of doublet microtubule organization and features specific to vertebrate cilia, including previously unknown MIPs, a luminal bundle of tektin filaments, and a pentameric dynein-docking complex. We identify a mechanism for bridging 48- to 24-nm periodicity across the microtubule wall and show that loss of the proteins involved causes defective ciliary motility and laterality abnormalities in zebrafish and mice. Our structure identifies candidate genes for diagnosis of ciliopathies and provides a framework to understand their functions in driving ciliary motility.
Dynein-decorated doublet microtubules (DMTs) are critical components of the oscillatory molecular machine of cilia, the axoneme, and have luminal surfaces patterned periodically by microtubule inner proteins (MIPs). Here we present an atomic model of the 48-nm repeat of a mammalian DMT, derived from a cryoelectron microscopy (cryo-EM) map of the complex isolated from bovine respiratory cilia. The structure uncovers principles of doublet microtubule organization and features specific to vertebrate cilia, including previously unknown MIPs, a luminal bundle of tektin filaments, and a pentameric dynein-docking complex. We identify a mechanism for bridging 48- to 24-nm periodicity across the microtubule wall and show that loss of the proteins involved causes defective ciliary motility and laterality abnormalities in zebrafish and mice. Our structure identifies candidate genes for diagnosis of ciliopathies and provides a framework to understand their functions in driving ciliary motility.
