EMD-26080
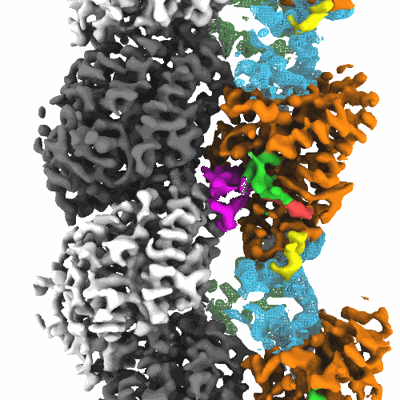
CaKip3[2-482] - AMP-PNP in complex with a dolastatin-10-stabilized tubulin ring
EMD-26080
Single-particle3.9 Å
 Deposition: 27/01/2022
Deposition: 27/01/2022Map released: 20/07/2022
Last modified: 21/02/2024
Sample Organism:
Sus scrofa,
Candida albicans
Sample: caKip3[2-482] - AMP-PNP in complex with a dolastatin-10-stabilized tubulin ring
Fitted models: 7tr3 (Avg. Q-score: 0.398)
Deposition Authors: Benoit MPMH ,
Asenjo AB
,
Asenjo AB  ,
Hunter B
,
Hunter B  ,
Allingham JS
,
Allingham JS  ,
Sosa H
,
Sosa H 
Sample: caKip3[2-482] - AMP-PNP in complex with a dolastatin-10-stabilized tubulin ring
Fitted models: 7tr3 (Avg. Q-score: 0.398)
Deposition Authors: Benoit MPMH
 ,
Asenjo AB
,
Asenjo AB  ,
Hunter B
,
Hunter B  ,
Allingham JS
,
Allingham JS  ,
Sosa H
,
Sosa H 
Kinesin-8-specific loop-2 controls the dual activities of the motor domain according to tubulin protofilament shape.
Hunter B  ,
Benoit MPMH
,
Benoit MPMH  ,
Asenjo AB
,
Asenjo AB  ,
Doubleday C,
Trofimova D,
Frazer C
,
Doubleday C,
Trofimova D,
Frazer C  ,
Shoukat I,
Sosa H
,
Shoukat I,
Sosa H  ,
Allingham JS
,
Allingham JS 
(2022) Nat Commun , 13 , 4198 - 4198
 ,
Benoit MPMH
,
Benoit MPMH  ,
Asenjo AB
,
Asenjo AB  ,
Doubleday C,
Trofimova D,
Frazer C
,
Doubleday C,
Trofimova D,
Frazer C  ,
Shoukat I,
Sosa H
,
Shoukat I,
Sosa H  ,
Allingham JS
,
Allingham JS 
(2022) Nat Commun , 13 , 4198 - 4198
Abstract:
Kinesin-8s are dual-activity motor proteins that can move processively on microtubules and depolymerize microtubule plus-ends, but their mechanism of combining these distinct activities remains unclear. We addressed this by obtaining cryo-EM structures (2.6-3.9 Å) of Candida albicans Kip3 in different catalytic states on the microtubule lattice and on a curved microtubule end mimic. We also determined a crystal structure of microtubule-unbound CaKip3-ADP (2.0 Å) and analyzed the biochemical activity of CaKip3 and kinesin-1 mutants. These data reveal that the microtubule depolymerization activity of kinesin-8 originates from conformational changes of its motor core that are amplified by dynamic contacts between its extended loop-2 and tubulin. On curved microtubule ends, loop-1 inserts into preceding motor domains, forming head-to-tail arrays of kinesin-8s that complement loop-2 contacts with curved tubulin and assist depolymerization. On straight tubulin protofilaments in the microtubule lattice, loop-2-tubulin contacts inhibit conformational changes in the motor core, but in the ADP-Pi state these contacts are relaxed, allowing neck-linker docking for motility. We propose that these tubulin shape-induced alternations between pro-microtubule-depolymerization and pro-motility kinesin states, regulated by loop-2, are the key to the dual activity of kinesin-8 motors.
Kinesin-8s are dual-activity motor proteins that can move processively on microtubules and depolymerize microtubule plus-ends, but their mechanism of combining these distinct activities remains unclear. We addressed this by obtaining cryo-EM structures (2.6-3.9 Å) of Candida albicans Kip3 in different catalytic states on the microtubule lattice and on a curved microtubule end mimic. We also determined a crystal structure of microtubule-unbound CaKip3-ADP (2.0 Å) and analyzed the biochemical activity of CaKip3 and kinesin-1 mutants. These data reveal that the microtubule depolymerization activity of kinesin-8 originates from conformational changes of its motor core that are amplified by dynamic contacts between its extended loop-2 and tubulin. On curved microtubule ends, loop-1 inserts into preceding motor domains, forming head-to-tail arrays of kinesin-8s that complement loop-2 contacts with curved tubulin and assist depolymerization. On straight tubulin protofilaments in the microtubule lattice, loop-2-tubulin contacts inhibit conformational changes in the motor core, but in the ADP-Pi state these contacts are relaxed, allowing neck-linker docking for motility. We propose that these tubulin shape-induced alternations between pro-microtubule-depolymerization and pro-motility kinesin states, regulated by loop-2, are the key to the dual activity of kinesin-8 motors.
