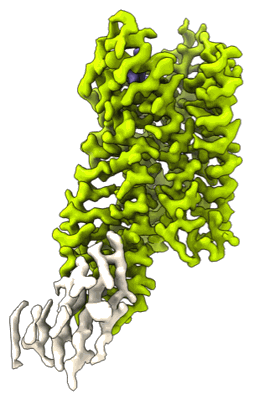
EMD-26589
CryoEM Structure of Inactive NTSR1 Bound to SR48692 and Nb6
EMD-26589
Single-particle2.4 Å
 Deposition: 03/04/2022
Deposition: 03/04/2022Map released: 29/06/2022
Last modified: 06/11/2024
Sample Organism:
Homo sapiens,
Lama glama
Sample: Complex of NTSR1 and Nb6
Fitted models: 7ul2 (Avg. Q-score: 0.645)
Raw data: EMPIAR-11136
Deposition Authors: Robertson MJ ,
Skiniotis G
,
Skiniotis G 
Sample: Complex of NTSR1 and Nb6
Fitted models: 7ul2 (Avg. Q-score: 0.645)
Raw data: EMPIAR-11136
Deposition Authors: Robertson MJ
 ,
Skiniotis G
,
Skiniotis G 
Structure determination of inactive-state GPCRs with a universal nanobody.
Robertson MJ  ,
Papasergi-Scott MM,
He F
,
Papasergi-Scott MM,
He F  ,
Seven AB,
Meyerowitz JG,
Panova O,
Peroto MC,
Che T
,
Seven AB,
Meyerowitz JG,
Panova O,
Peroto MC,
Che T  ,
Skiniotis G
,
Skiniotis G 
(2022) Nat Struct Mol Biol , 29 , 1188 - 1195
 ,
Papasergi-Scott MM,
He F
,
Papasergi-Scott MM,
He F  ,
Seven AB,
Meyerowitz JG,
Panova O,
Peroto MC,
Che T
,
Seven AB,
Meyerowitz JG,
Panova O,
Peroto MC,
Che T  ,
Skiniotis G
,
Skiniotis G 
(2022) Nat Struct Mol Biol , 29 , 1188 - 1195
Abstract:
Cryogenic electron microscopy (cryo-EM) has widened the field of structure-based drug discovery by allowing for routine determination of membrane protein structures previously intractable. Despite representing one of the largest classes of therapeutic targets, most inactive-state G protein-coupled receptors (GPCRs) have remained inaccessible for cryo-EM because their small size and membrane-embedded nature impedes projection alignment for high-resolution map reconstructions. Here we demonstrate that the same single-chain camelid antibody (nanobody) recognizing a grafted intracellular loop can be used to obtain cryo-EM structures of inactive-state GPCRs at resolutions comparable or better than those obtained by X-ray crystallography. Using this approach, we obtained structures of neurotensin 1 receptor bound to antagonist SR48692, μ-opioid receptor bound to alvimopan, apo somatostatin receptor 2 and histamine receptor 2 bound to famotidine. We expect this rapid, straightforward approach to facilitate the broad exploration of GPCR inactive states without the need for extensive engineering and crystallization.
Cryogenic electron microscopy (cryo-EM) has widened the field of structure-based drug discovery by allowing for routine determination of membrane protein structures previously intractable. Despite representing one of the largest classes of therapeutic targets, most inactive-state G protein-coupled receptors (GPCRs) have remained inaccessible for cryo-EM because their small size and membrane-embedded nature impedes projection alignment for high-resolution map reconstructions. Here we demonstrate that the same single-chain camelid antibody (nanobody) recognizing a grafted intracellular loop can be used to obtain cryo-EM structures of inactive-state GPCRs at resolutions comparable or better than those obtained by X-ray crystallography. Using this approach, we obtained structures of neurotensin 1 receptor bound to antagonist SR48692, μ-opioid receptor bound to alvimopan, apo somatostatin receptor 2 and histamine receptor 2 bound to famotidine. We expect this rapid, straightforward approach to facilitate the broad exploration of GPCR inactive states without the need for extensive engineering and crystallization.
