EMD-27638
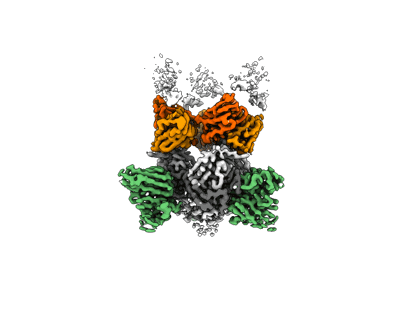
Structure of EBOV GP lacking the mucin-like domain with 9.20.1A2 Fab and 6D6 scFv bound
EMD-27638
Single-particle3.0 Å
 Deposition: 15/07/2022
Deposition: 15/07/2022Map released: 19/07/2023
Last modified: 13/11/2024
Sample Organism:
Ebola virus - Mayinga, Zaire, 1976,
Homo sapiens,
Mus musculus
Sample: Complex of Ebola virus glycoprotein trimer with 1A2 antibody Fab and 6D6 antibody scFv
Fitted models: 8dpm (Avg. Q-score: 0.505)
Deposition Authors: Yu X ,
Saphire EO
,
Saphire EO 
Sample: Complex of Ebola virus glycoprotein trimer with 1A2 antibody Fab and 6D6 antibody scFv
Fitted models: 8dpm (Avg. Q-score: 0.505)
Deposition Authors: Yu X
 ,
Saphire EO
,
Saphire EO 
The evolution and determinants of neutralization of potent head-binding antibodies against Ebola virus.
Yu X  ,
Hastie KM,
Davis CW,
Avalos RD,
Williams D,
Parekh D,
Hui S
,
Hastie KM,
Davis CW,
Avalos RD,
Williams D,
Parekh D,
Hui S  ,
Mann C,
Hariharan C,
Takada A,
Ahmed R,
Saphire EO
,
Mann C,
Hariharan C,
Takada A,
Ahmed R,
Saphire EO 
(2023) Cell Rep , 42 , 113366 - 113366
 ,
Hastie KM,
Davis CW,
Avalos RD,
Williams D,
Parekh D,
Hui S
,
Hastie KM,
Davis CW,
Avalos RD,
Williams D,
Parekh D,
Hui S  ,
Mann C,
Hariharan C,
Takada A,
Ahmed R,
Saphire EO
,
Mann C,
Hariharan C,
Takada A,
Ahmed R,
Saphire EO 
(2023) Cell Rep , 42 , 113366 - 113366
Abstract:
Monoclonal antibodies against the Ebola virus (EBOV) surface glycoprotein are effective treatments for EBOV disease. Antibodies targeting the EBOV glycoprotein (GP) head epitope have potent neutralization and Fc effector function activity and thus are of high interest as therapeutics and for vaccine design. Here we focus on the head-binding antibodies 1A2 and 1D5, which have been identified previously in a longitudinal study of survivors of EBOV infection. 1A2 and 1D5 have the same heavy- and light-chain germlines despite being isolated from different individuals and at different time points after recovery from infection. Cryoelectron microscopy analysis of each antibody in complex with the EBOV surface GP reveals key amino acid substitutions in 1A2 that contribute to greater affinity, improved neutralization potency, and enhanced breadth as well as two strategies for antibody evolution from a common site.
Monoclonal antibodies against the Ebola virus (EBOV) surface glycoprotein are effective treatments for EBOV disease. Antibodies targeting the EBOV glycoprotein (GP) head epitope have potent neutralization and Fc effector function activity and thus are of high interest as therapeutics and for vaccine design. Here we focus on the head-binding antibodies 1A2 and 1D5, which have been identified previously in a longitudinal study of survivors of EBOV infection. 1A2 and 1D5 have the same heavy- and light-chain germlines despite being isolated from different individuals and at different time points after recovery from infection. Cryoelectron microscopy analysis of each antibody in complex with the EBOV surface GP reveals key amino acid substitutions in 1A2 that contribute to greater affinity, improved neutralization potency, and enhanced breadth as well as two strategies for antibody evolution from a common site.
