EMD-28049
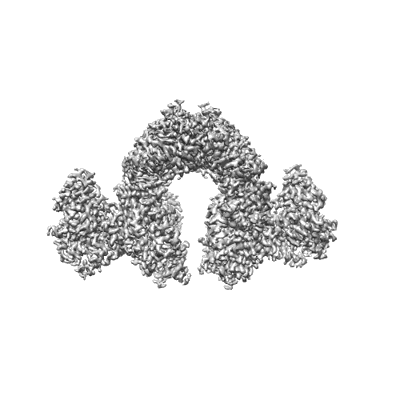
Structure of E.coli Septu (PtuAB) complex
EMD-28049
Composite mapSingle-particle
2.6 Å
 Deposition: 06/09/2022
Deposition: 06/09/2022Map released: 27/12/2023
Last modified: 03/04/2024
Sample Organism:
Escherichia coli
Sample: 6 PtuA and 2 PtuB form as a complex
Fitted models: 8eea (Avg. Q-score: 0.603)
Deposition Authors: Shen ZF, Fu TM
Sample: 6 PtuA and 2 PtuB form as a complex
Fitted models: 8eea (Avg. Q-score: 0.603)
Deposition Authors: Shen ZF, Fu TM

PtuA and PtuB assemble into an inflammasome-like oligomer for anti-phage defense.
Li Y,
Shen Z  ,
Zhang M,
Yang XY
,
Zhang M,
Yang XY  ,
Cleary SP,
Xie J
,
Cleary SP,
Xie J  ,
Marathe IA,
Kostelic M,
Greenwald J
,
Marathe IA,
Kostelic M,
Greenwald J  ,
Rish AD
,
Rish AD  ,
Wysocki VH
,
Wysocki VH  ,
Chen C,
Chen Q
,
Chen C,
Chen Q  ,
Fu TM
,
Fu TM  ,
Yu Y
,
Yu Y 
(2024) Nat Struct Mol Biol , 31 , 413 - 423
 ,
Zhang M,
Yang XY
,
Zhang M,
Yang XY  ,
Cleary SP,
Xie J
,
Cleary SP,
Xie J  ,
Marathe IA,
Kostelic M,
Greenwald J
,
Marathe IA,
Kostelic M,
Greenwald J  ,
Rish AD
,
Rish AD  ,
Wysocki VH
,
Wysocki VH  ,
Chen C,
Chen Q
,
Chen C,
Chen Q  ,
Fu TM
,
Fu TM  ,
Yu Y
,
Yu Y 
(2024) Nat Struct Mol Biol , 31 , 413 - 423
Abstract:
Escherichia coli Septu system, an anti-phage defense system, comprises two components: PtuA and PtuB. PtuA contains an ATPase domain, while PtuB is predicted to function as a nuclease. Here we show that PtuA and PtuB form a stable complex with a 6:2 stoichiometry. Cryo-electron microscopy structure of PtuAB reveals a distinctive horseshoe-like configuration. PtuA adopts a hexameric arrangement, organized as an asymmetric trimer of dimers, contrasting the ring-like structure by other ATPases. Notably, the three pairs of PtuA dimers assume distinct conformations and fulfill unique roles in recruiting PtuB. Our functional assays have further illuminated the importance of the oligomeric assembly of PtuAB in anti-phage defense. Moreover, we have uncovered that ATP molecules can directly bind to PtuA and inhibit the activities of PtuAB. Together, the assembly and function of the Septu system shed light on understanding other ATPase-containing systems in bacterial immunity.
Escherichia coli Septu system, an anti-phage defense system, comprises two components: PtuA and PtuB. PtuA contains an ATPase domain, while PtuB is predicted to function as a nuclease. Here we show that PtuA and PtuB form a stable complex with a 6:2 stoichiometry. Cryo-electron microscopy structure of PtuAB reveals a distinctive horseshoe-like configuration. PtuA adopts a hexameric arrangement, organized as an asymmetric trimer of dimers, contrasting the ring-like structure by other ATPases. Notably, the three pairs of PtuA dimers assume distinct conformations and fulfill unique roles in recruiting PtuB. Our functional assays have further illuminated the importance of the oligomeric assembly of PtuAB in anti-phage defense. Moreover, we have uncovered that ATP molecules can directly bind to PtuA and inhibit the activities of PtuAB. Together, the assembly and function of the Septu system shed light on understanding other ATPase-containing systems in bacterial immunity.
