EMD-29599
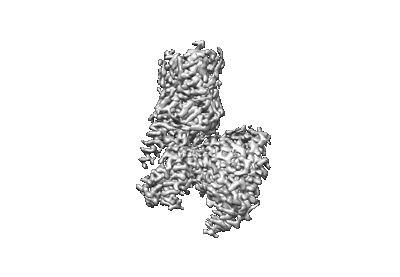
Buspirone-bound serotonin 1A (5-HT1A) receptor-Gi1 protein complex
EMD-29599
Single-particle2.62 Å
 Deposition: 26/01/2023
Deposition: 26/01/2023Map released: 15/05/2024
Last modified: 06/11/2024
Sample Organism:
Homo sapiens,
Escherichia coli, Homo sapiens
Sample: Serotonin 1A (5-HT1A) receptor-Gi1 protein complex
Fitted models: 8fyx (Avg. Q-score: 0.545)
Deposition Authors: Warren AL ,
Zilberg G
,
Zilberg G  ,
Capper MJ
,
Capper MJ  ,
Wacker D
,
Wacker D 
Sample: Serotonin 1A (5-HT1A) receptor-Gi1 protein complex
Fitted models: 8fyx (Avg. Q-score: 0.545)
Deposition Authors: Warren AL
 ,
Zilberg G
,
Zilberg G  ,
Capper MJ
,
Capper MJ  ,
Wacker D
,
Wacker D 
Structural pharmacology and therapeutic potential of 5-methoxytryptamines.
Warren AL  ,
Lankri D
,
Lankri D  ,
Cunningham MJ
,
Cunningham MJ  ,
Serrano IC
,
Serrano IC  ,
Parise LF
,
Parise LF  ,
Kruegel AC,
Duggan P
,
Kruegel AC,
Duggan P  ,
Zilberg G
,
Zilberg G  ,
Capper MJ
,
Capper MJ  ,
Havel V
,
Havel V  ,
Russo SJ
,
Russo SJ  ,
Sames D
,
Sames D  ,
Wacker D
,
Wacker D 
(2024) Nature , 630 , 237 - 246
 ,
Lankri D
,
Lankri D  ,
Cunningham MJ
,
Cunningham MJ  ,
Serrano IC
,
Serrano IC  ,
Parise LF
,
Parise LF  ,
Kruegel AC,
Duggan P
,
Kruegel AC,
Duggan P  ,
Zilberg G
,
Zilberg G  ,
Capper MJ
,
Capper MJ  ,
Havel V
,
Havel V  ,
Russo SJ
,
Russo SJ  ,
Sames D
,
Sames D  ,
Wacker D
,
Wacker D 
(2024) Nature , 630 , 237 - 246
Abstract:
Psychedelic substances such as lysergic acid diethylamide (LSD) and psilocybin show potential for the treatment of various neuropsychiatric disorders1-3. These compounds are thought to mediate their hallucinogenic and therapeutic effects through the serotonin (5-hydroxytryptamine (5-HT)) receptor 5-HT2A (ref. 4). However, 5-HT1A also plays a part in the behavioural effects of tryptamine hallucinogens5, particularly 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), a psychedelic found in the toxin of Colorado River toads6. Although 5-HT1A is a validated therapeutic target7,8, little is known about how psychedelics engage 5-HT1A and which effects are mediated by this receptor. Here we map the molecular underpinnings of 5-MeO-DMT pharmacology through five cryogenic electron microscopy (cryo-EM) structures of 5-HT1A, systematic medicinal chemistry, receptor mutagenesis and mouse behaviour. Structure-activity relationship analyses of 5-methoxytryptamines at both 5-HT1A and 5-HT2A enable the characterization of molecular determinants of 5-HT1A signalling potency, efficacy and selectivity. Moreover, we contrast the structural interactions and in vitro pharmacology of 5-MeO-DMT and analogues to the pan-serotonergic agonist LSD and clinically used 5-HT1A agonists. We show that a 5-HT1A-selective 5-MeO-DMT analogue is devoid of hallucinogenic-like effects while retaining anxiolytic-like and antidepressant-like activity in socially defeated animals. Our studies uncover molecular aspects of 5-HT1A-targeted psychedelics and therapeutics, which may facilitate the future development of new medications for neuropsychiatric disorders.
Psychedelic substances such as lysergic acid diethylamide (LSD) and psilocybin show potential for the treatment of various neuropsychiatric disorders1-3. These compounds are thought to mediate their hallucinogenic and therapeutic effects through the serotonin (5-hydroxytryptamine (5-HT)) receptor 5-HT2A (ref. 4). However, 5-HT1A also plays a part in the behavioural effects of tryptamine hallucinogens5, particularly 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), a psychedelic found in the toxin of Colorado River toads6. Although 5-HT1A is a validated therapeutic target7,8, little is known about how psychedelics engage 5-HT1A and which effects are mediated by this receptor. Here we map the molecular underpinnings of 5-MeO-DMT pharmacology through five cryogenic electron microscopy (cryo-EM) structures of 5-HT1A, systematic medicinal chemistry, receptor mutagenesis and mouse behaviour. Structure-activity relationship analyses of 5-methoxytryptamines at both 5-HT1A and 5-HT2A enable the characterization of molecular determinants of 5-HT1A signalling potency, efficacy and selectivity. Moreover, we contrast the structural interactions and in vitro pharmacology of 5-MeO-DMT and analogues to the pan-serotonergic agonist LSD and clinically used 5-HT1A agonists. We show that a 5-HT1A-selective 5-MeO-DMT analogue is devoid of hallucinogenic-like effects while retaining anxiolytic-like and antidepressant-like activity in socially defeated animals. Our studies uncover molecular aspects of 5-HT1A-targeted psychedelics and therapeutics, which may facilitate the future development of new medications for neuropsychiatric disorders.
