EMD-34406
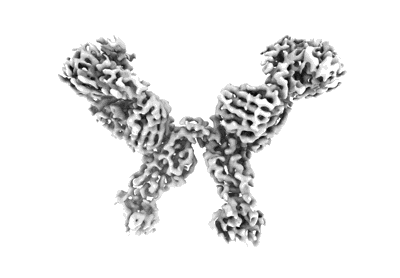
Local refinement of SARS-CoV-2 Omicron BA.1 Spike glycoprotein in complex with rabbit monoclonal antibody 1H1 Fab
EMD-34406
Single-particle3.52 Å
 Deposition: 27/09/2022
Deposition: 27/09/2022Map released: 12/04/2023
Last modified: 13/11/2024
Sample Organism:
Severe acute respiratory syndrome coronavirus 2,
Oryctolagus cuniculus
Sample: SARS-CoV-2 Omicron spike glycoprotein complex with 55A8 Fab
Fitted models: 8gzz (Avg. Q-score: 0.491)
Deposition Authors: Guo H ,
Gao Y
,
Gao Y  ,
Lu Y
,
Lu Y  ,
Yang H
,
Yang H  ,
Ji X
,
Ji X 
Sample: SARS-CoV-2 Omicron spike glycoprotein complex with 55A8 Fab
Fitted models: 8gzz (Avg. Q-score: 0.491)
Deposition Authors: Guo H
 ,
Gao Y
,
Gao Y  ,
Lu Y
,
Lu Y  ,
Yang H
,
Yang H  ,
Ji X
,
Ji X 
Mechanism of a rabbit monoclonal antibody broadly neutralizing SARS-CoV-2 variants.
Guo H  ,
Yang Y,
Zhao T,
Lu Y
,
Yang Y,
Zhao T,
Lu Y  ,
Gao Y
,
Gao Y  ,
Li T,
Xiao H,
Chu X,
Zheng L,
Li W,
Cheng H,
Huang H,
Liu Y,
Lou Y,
Nguyen HC,
Wu C,
Chen Y
,
Li T,
Xiao H,
Chu X,
Zheng L,
Li W,
Cheng H,
Huang H,
Liu Y,
Lou Y,
Nguyen HC,
Wu C,
Chen Y  ,
Yang H
,
Yang H  ,
Ji X
,
Ji X 
(2023) Commun Biol , 6 , 364 - 364
 ,
Yang Y,
Zhao T,
Lu Y
,
Yang Y,
Zhao T,
Lu Y  ,
Gao Y
,
Gao Y  ,
Li T,
Xiao H,
Chu X,
Zheng L,
Li W,
Cheng H,
Huang H,
Liu Y,
Lou Y,
Nguyen HC,
Wu C,
Chen Y
,
Li T,
Xiao H,
Chu X,
Zheng L,
Li W,
Cheng H,
Huang H,
Liu Y,
Lou Y,
Nguyen HC,
Wu C,
Chen Y  ,
Yang H
,
Yang H  ,
Ji X
,
Ji X 
(2023) Commun Biol , 6 , 364 - 364
Abstract:
Due to the continuous evolution of SARS-CoV-2, the Omicron variant has emerged and exhibits severe immune evasion. The high number of mutations at key antigenic sites on the spike protein has made a large number of existing antibodies and vaccines ineffective against this variant. Therefore, it is urgent to develop efficient broad-spectrum neutralizing therapeutic drugs. Here we characterize a rabbit monoclonal antibody (RmAb) 1H1 with broad-spectrum neutralizing potency against Omicron sublineages including BA.1, BA.1.1, BA.2, BA.2.12.1, BA.2.75, BA.3 and BA.4/5. Cryo-electron microscopy (cryo-EM) structure determination of the BA.1 spike-1H1 Fab complexes shows that 1H1 targets a highly conserved region of RBD and avoids most of the circulating Omicron mutations, explaining its broad-spectrum neutralization potency. Our findings indicate 1H1 as a promising RmAb model for designing broad-spectrum neutralizing antibodies and shed light on the development of therapeutic agents as well as effective vaccines against newly emerging variants in the future.
Due to the continuous evolution of SARS-CoV-2, the Omicron variant has emerged and exhibits severe immune evasion. The high number of mutations at key antigenic sites on the spike protein has made a large number of existing antibodies and vaccines ineffective against this variant. Therefore, it is urgent to develop efficient broad-spectrum neutralizing therapeutic drugs. Here we characterize a rabbit monoclonal antibody (RmAb) 1H1 with broad-spectrum neutralizing potency against Omicron sublineages including BA.1, BA.1.1, BA.2, BA.2.12.1, BA.2.75, BA.3 and BA.4/5. Cryo-electron microscopy (cryo-EM) structure determination of the BA.1 spike-1H1 Fab complexes shows that 1H1 targets a highly conserved region of RBD and avoids most of the circulating Omicron mutations, explaining its broad-spectrum neutralization potency. Our findings indicate 1H1 as a promising RmAb model for designing broad-spectrum neutralizing antibodies and shed light on the development of therapeutic agents as well as effective vaccines against newly emerging variants in the future.
