EMD-36339
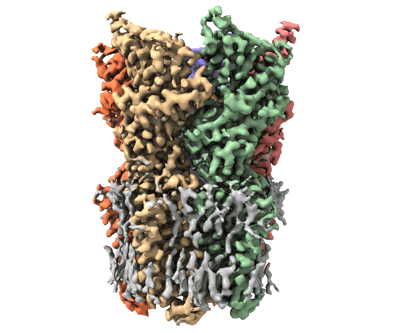
Cryo-EM structure of nanodisc (PE:PS:PC) reconstituted GLIC at pH 2.5
EMD-36339
Single-particle2.6476 Å
 Deposition: 29/05/2023
Deposition: 29/05/2023Map released: 17/04/2024
Last modified: 17/04/2024
Sample Organism:
Gloeobacter violaceus
Sample: pentameric ligand-gated ion channel
Fitted models: 8jj3 (Avg. Q-score: 0.624)
Deposition Authors: Bharambe N ,
Li Z
,
Li Z  ,
Basak S
,
Basak S 
Sample: pentameric ligand-gated ion channel
Fitted models: 8jj3 (Avg. Q-score: 0.624)
Deposition Authors: Bharambe N
 ,
Li Z
,
Li Z  ,
Basak S
,
Basak S 
Cryo-EM structures of prokaryotic ligand-gated ion channel GLIC provide insights into gating in a lipid environment.
Bharambe N  ,
Li Z
,
Li Z  ,
Seiferth D
,
Seiferth D  ,
Balakrishna AM
,
Balakrishna AM  ,
Biggin PC
,
Biggin PC  ,
Basak S
,
Basak S 
(2024) Nat Commun , 15 , 2967 - 2967
 ,
Li Z
,
Li Z  ,
Seiferth D
,
Seiferth D  ,
Balakrishna AM
,
Balakrishna AM  ,
Biggin PC
,
Biggin PC  ,
Basak S
,
Basak S 
(2024) Nat Commun , 15 , 2967 - 2967
Abstract:
GLIC, a proton-activated prokaryotic ligand-gated ion channel, served as a model system for understanding the eukaryotic counterparts due to their structural and functional similarities. Despite extensive studies conducted on GLIC, the molecular mechanism of channel gating in the lipid environment requires further investigation. Here, we present the cryo-EM structures of nanodisc-reconstituted GLIC at neutral and acidic pH in the resolution range of 2.6 - 3.4 Å. In our apo state at pH 7.5, the extracellular domain (ECD) displays conformational variations compared to the existing apo structures. At pH 4.0, three distinct conformational states (C1, C2 and O states) are identified. The protonated structures exhibit a compacted and counter-clockwise rotated ECD compared with our apo state. A gradual widening of the pore in the TMD is observed upon reducing the pH, with the widest pore in O state, accompanied by several layers of water pentagons. The pore radius and molecular dynamics (MD) simulations suggest that the O state represents an open conductive state. We also observe state-dependent interactions between several lipids and proteins that may be involved in the regulation of channel gating. Our results provide comprehensive insights into the importance of lipids impact on gating.
GLIC, a proton-activated prokaryotic ligand-gated ion channel, served as a model system for understanding the eukaryotic counterparts due to their structural and functional similarities. Despite extensive studies conducted on GLIC, the molecular mechanism of channel gating in the lipid environment requires further investigation. Here, we present the cryo-EM structures of nanodisc-reconstituted GLIC at neutral and acidic pH in the resolution range of 2.6 - 3.4 Å. In our apo state at pH 7.5, the extracellular domain (ECD) displays conformational variations compared to the existing apo structures. At pH 4.0, three distinct conformational states (C1, C2 and O states) are identified. The protonated structures exhibit a compacted and counter-clockwise rotated ECD compared with our apo state. A gradual widening of the pore in the TMD is observed upon reducing the pH, with the widest pore in O state, accompanied by several layers of water pentagons. The pore radius and molecular dynamics (MD) simulations suggest that the O state represents an open conductive state. We also observe state-dependent interactions between several lipids and proteins that may be involved in the regulation of channel gating. Our results provide comprehensive insights into the importance of lipids impact on gating.
