Happy Holidays & Happy New Year from the EMDB team! During this period (23rd December - 6th January) the EMDB team and our OneDep curators will be working at reduced capacity. We will continue to run the weekly release of entries as normal, but entry annotation & support may be slower than usual. Thanks for your understanding.
EMD-41431
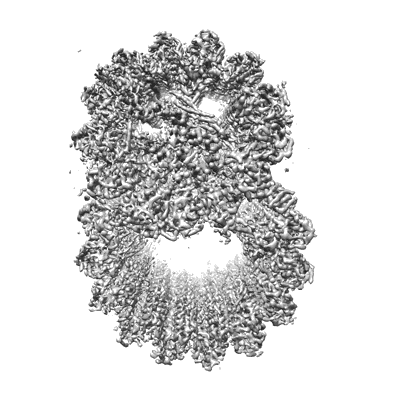
48-nm repeating structure of doublets from mouse sperm flagella
EMD-41431
Subtomogram averaging7.7 Å
 Deposition: 02/08/2023
Deposition: 02/08/2023Map released: 01/11/2023
Last modified: 22/11/2023
Sample Organism:
Mus musculus
Sample: Mouse sperm
Fitted models: 8to0
Deposition Authors: Chen Z ,
Shiozak M,
Hass KM,
Skinner W,
Zhao S,
Guo C,
Polacco BJ,
Yu Z,
Krogan NJ,
Kaake RM,
Vale RD,
Agard DA
,
Shiozak M,
Hass KM,
Skinner W,
Zhao S,
Guo C,
Polacco BJ,
Yu Z,
Krogan NJ,
Kaake RM,
Vale RD,
Agard DA 
Sample: Mouse sperm
Fitted models: 8to0
Deposition Authors: Chen Z
 ,
Shiozak M,
Hass KM,
Skinner W,
Zhao S,
Guo C,
Polacco BJ,
Yu Z,
Krogan NJ,
Kaake RM,
Vale RD,
Agard DA
,
Shiozak M,
Hass KM,
Skinner W,
Zhao S,
Guo C,
Polacco BJ,
Yu Z,
Krogan NJ,
Kaake RM,
Vale RD,
Agard DA 
De novo protein identification in mammalian sperm using in situ cryoelectron tomography and AlphaFold2 docking.
Chen Z  ,
Shiozaki M,
Haas KM
,
Shiozaki M,
Haas KM  ,
Skinner WM,
Zhao S,
Guo C,
Polacco BJ,
Yu Z,
Krogan NJ,
Lishko PV,
Kaake RM,
Vale RD,
Agard DA
,
Skinner WM,
Zhao S,
Guo C,
Polacco BJ,
Yu Z,
Krogan NJ,
Lishko PV,
Kaake RM,
Vale RD,
Agard DA 
(2023) Cell , 186 , 5041 - 5053.e19
 ,
Shiozaki M,
Haas KM
,
Shiozaki M,
Haas KM  ,
Skinner WM,
Zhao S,
Guo C,
Polacco BJ,
Yu Z,
Krogan NJ,
Lishko PV,
Kaake RM,
Vale RD,
Agard DA
,
Skinner WM,
Zhao S,
Guo C,
Polacco BJ,
Yu Z,
Krogan NJ,
Lishko PV,
Kaake RM,
Vale RD,
Agard DA 
(2023) Cell , 186 , 5041 - 5053.e19
Abstract:
To understand the molecular mechanisms of cellular pathways, contemporary workflows typically require multiple techniques to identify proteins, track their localization, and determine their structures in vitro. Here, we combined cellular cryoelectron tomography (cryo-ET) and AlphaFold2 modeling to address these questions and understand how mammalian sperm are built in situ. Our cellular cryo-ET and subtomogram averaging provided 6.0-Å reconstructions of axonemal microtubule structures. The well-resolved tertiary structures allowed us to unbiasedly match sperm-specific densities with 21,615 AlphaFold2-predicted protein models of the mouse proteome. We identified Tektin 5, CCDC105, and SPACA9 as novel microtubule-associated proteins. These proteins form an extensive interaction network crosslinking the lumen of axonemal doublet microtubules, suggesting their roles in modulating the mechanical properties of the filaments. Indeed, Tekt5 -/- sperm possess more deformed flagella with 180° bends. Together, our studies presented a cellular visual proteomics workflow and shed light on the in vivo functions of Tektin 5.
To understand the molecular mechanisms of cellular pathways, contemporary workflows typically require multiple techniques to identify proteins, track their localization, and determine their structures in vitro. Here, we combined cellular cryoelectron tomography (cryo-ET) and AlphaFold2 modeling to address these questions and understand how mammalian sperm are built in situ. Our cellular cryo-ET and subtomogram averaging provided 6.0-Å reconstructions of axonemal microtubule structures. The well-resolved tertiary structures allowed us to unbiasedly match sperm-specific densities with 21,615 AlphaFold2-predicted protein models of the mouse proteome. We identified Tektin 5, CCDC105, and SPACA9 as novel microtubule-associated proteins. These proteins form an extensive interaction network crosslinking the lumen of axonemal doublet microtubules, suggesting their roles in modulating the mechanical properties of the filaments. Indeed, Tekt5 -/- sperm possess more deformed flagella with 180° bends. Together, our studies presented a cellular visual proteomics workflow and shed light on the in vivo functions of Tektin 5.
