EMD-42015
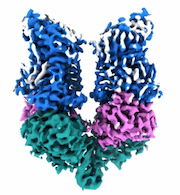
Structural Basis of Human NOX5 Activation
EMD-42015
Single-particle3.3 Å
 Deposition: 15/09/2023
Deposition: 15/09/2023Map released: 01/05/2024
Last modified: 06/11/2024
Sample Organism:
Homo sapiens
Sample: NADPH oxidase 5
Fitted models: 8u86 (Avg. Q-score: 0.45)
Deposition Authors: Cui C ,
Jiang M,
Sun J
,
Jiang M,
Sun J 
Sample: NADPH oxidase 5
Fitted models: 8u86 (Avg. Q-score: 0.45)
Deposition Authors: Cui C
 ,
Jiang M,
Sun J
,
Jiang M,
Sun J 
Structural basis of human NOX5 activation.
Cui C  ,
Jiang M,
Jain N,
Das S
,
Jiang M,
Jain N,
Das S  ,
Lo YH,
Kermani AA,
Pipatpolkai T
,
Lo YH,
Kermani AA,
Pipatpolkai T  ,
Sun J
,
Sun J 
(2024) Nat Commun , 15 , 3994 - 3994
 ,
Jiang M,
Jain N,
Das S
,
Jiang M,
Jain N,
Das S  ,
Lo YH,
Kermani AA,
Pipatpolkai T
,
Lo YH,
Kermani AA,
Pipatpolkai T  ,
Sun J
,
Sun J 
(2024) Nat Commun , 15 , 3994 - 3994
Abstract:
NADPH oxidase 5 (NOX5) catalyzes the production of superoxide free radicals and regulates physiological processes from sperm motility to cardiac rhythm. Overexpression of NOX5 leads to cancers, diabetes, and cardiovascular diseases. NOX5 is activated by intracellular calcium signaling, but the underlying molecular mechanism of which - in particular, how calcium triggers electron transfer from NADPH to FAD - is still unclear. Here we capture motions of full-length human NOX5 upon calcium binding using single-particle cryogenic electron microscopy (cryo-EM). By combining biochemistry, mutagenesis analyses, and molecular dynamics (MD) simulations, we decode the molecular basis of NOX5 activation and electron transfer. We find that calcium binding to the EF-hand domain increases NADPH dynamics, permitting electron transfer between NADPH and FAD and superoxide production. Our structural findings also uncover a zinc-binding motif that is important for NOX5 stability and enzymatic activity, revealing modulation mechanisms of reactive oxygen species (ROS) production.
NADPH oxidase 5 (NOX5) catalyzes the production of superoxide free radicals and regulates physiological processes from sperm motility to cardiac rhythm. Overexpression of NOX5 leads to cancers, diabetes, and cardiovascular diseases. NOX5 is activated by intracellular calcium signaling, but the underlying molecular mechanism of which - in particular, how calcium triggers electron transfer from NADPH to FAD - is still unclear. Here we capture motions of full-length human NOX5 upon calcium binding using single-particle cryogenic electron microscopy (cryo-EM). By combining biochemistry, mutagenesis analyses, and molecular dynamics (MD) simulations, we decode the molecular basis of NOX5 activation and electron transfer. We find that calcium binding to the EF-hand domain increases NADPH dynamics, permitting electron transfer between NADPH and FAD and superoxide production. Our structural findings also uncover a zinc-binding motif that is important for NOX5 stability and enzymatic activity, revealing modulation mechanisms of reactive oxygen species (ROS) production.
