EMD-42285
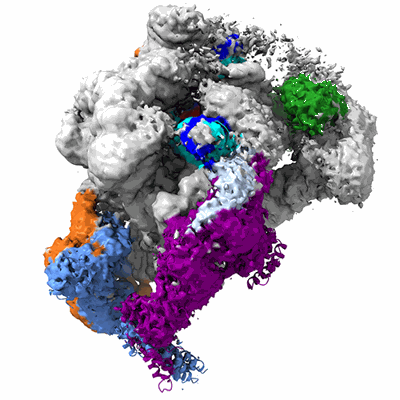
Structure of poised transcription complex Pol II-DSIF-NELF - pre-translocated
EMD-42285
Single-particle2.7 Å
 Deposition: 09/10/2023
Deposition: 09/10/2023Map released: 20/03/2024
Last modified: 11/09/2024
Sample Organism:
Sus scrofa,
Homo sapiens,
synthetic construct
Sample: RNA Polymerase in complex with DSIF and NELF
Fitted models: 8ui0 (Avg. Q-score: 0.352)
Deposition Authors: Vos SM, Su BG
Sample: RNA Polymerase in complex with DSIF and NELF
Fitted models: 8ui0 (Avg. Q-score: 0.352)
Deposition Authors: Vos SM, Su BG
Distinct negative elongation factor conformations regulate RNA polymerase II promoter-proximal pausing.
Abstract:
Metazoan gene expression regulation involves pausing of RNA polymerase (Pol II) in the promoter-proximal region of genes and is stabilized by DSIF and NELF. Upon depletion of elongation factors, NELF appears to accompany elongating Pol II past pause sites; however, prior work indicates that NELF prevents Pol II elongation. Here, we report cryoelectron microscopy structures of Pol II-DSIF-NELF complexes with NELF in two distinct conformations corresponding to paused and poised states. The paused NELF state supports Pol II stalling, whereas the poised NELF state enables transcription elongation as it does not support a tilted RNA-DNA hybrid. Further, the poised NELF state can accommodate TFIIS binding to Pol II, allowing for Pol II reactivation at paused or backtracking sites. Finally, we observe that the NELF-A tentacle interacts with the RPB2 protrusion and is necessary for pausing. Our results define how NELF can support pausing, reactivation, and elongation by Pol II.
Metazoan gene expression regulation involves pausing of RNA polymerase (Pol II) in the promoter-proximal region of genes and is stabilized by DSIF and NELF. Upon depletion of elongation factors, NELF appears to accompany elongating Pol II past pause sites; however, prior work indicates that NELF prevents Pol II elongation. Here, we report cryoelectron microscopy structures of Pol II-DSIF-NELF complexes with NELF in two distinct conformations corresponding to paused and poised states. The paused NELF state supports Pol II stalling, whereas the poised NELF state enables transcription elongation as it does not support a tilted RNA-DNA hybrid. Further, the poised NELF state can accommodate TFIIS binding to Pol II, allowing for Pol II reactivation at paused or backtracking sites. Finally, we observe that the NELF-A tentacle interacts with the RPB2 protrusion and is necessary for pausing. Our results define how NELF can support pausing, reactivation, and elongation by Pol II.
