EMD-43327
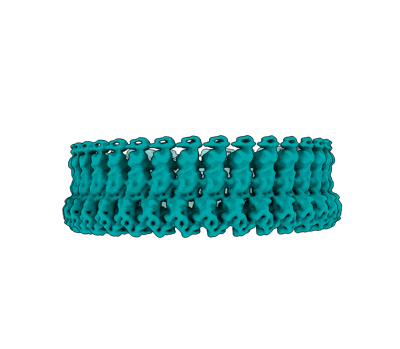
CW Flagellar Switch Complex - FliF, FliG, FliM, and FliN forming the C-ring from Salmonella
EMD-43327
Composite mapSingle-particle
4.6 Å
 Deposition: 09/01/2024
Deposition: 09/01/2024Map released: 28/02/2024
Last modified: 21/08/2024
Sample Organism:
Salmonella enterica subsp. enterica serovar Typhimurium
Sample: Flagellar C-ring containing FliF, FliG, FliM, and FliN
Fitted models: 8vkq (Avg. Q-score: 0.144)
Raw data: EMPIAR-11891, EMPIAR-11892
Deposition Authors: Singh PK ,
Iverson TM
,
Iverson TM 
Sample: Flagellar C-ring containing FliF, FliG, FliM, and FliN
Fitted models: 8vkq (Avg. Q-score: 0.144)
Raw data: EMPIAR-11891, EMPIAR-11892
Deposition Authors: Singh PK
 ,
Iverson TM
,
Iverson TM 
CryoEM structures reveal how the bacterial flagellum rotates and switches direction.
Singh PK  ,
Sharma P
,
Sharma P  ,
Afanzar O,
Goldfarb MH,
Maklashina E,
Eisenbach M
,
Afanzar O,
Goldfarb MH,
Maklashina E,
Eisenbach M  ,
Cecchini G
,
Cecchini G  ,
Iverson TM
,
Iverson TM 
(2024) Nat Microbiol , 9 , 1271 - 1281
 ,
Sharma P
,
Sharma P  ,
Afanzar O,
Goldfarb MH,
Maklashina E,
Eisenbach M
,
Afanzar O,
Goldfarb MH,
Maklashina E,
Eisenbach M  ,
Cecchini G
,
Cecchini G  ,
Iverson TM
,
Iverson TM 
(2024) Nat Microbiol , 9 , 1271 - 1281
Abstract:
Bacterial chemotaxis requires bidirectional flagellar rotation at different rates. Rotation is driven by a flagellar motor, which is a supercomplex containing multiple rings. Architectural uncertainty regarding the cytoplasmic C-ring, or 'switch', limits our understanding of how the motor transmits torque and direction to the flagellar rod. Here we report cryogenic electron microscopy structures for Salmonella enterica serovar typhimurium inner membrane MS-ring and C-ring in a counterclockwise pose (4.0 Å) and isolated C-ring in a clockwise pose alone (4.6 Å) and bound to a regulator (5.9 Å). Conformational differences between rotational poses include a 180° shift in FliF/FliG domains that rotates the outward-facing MotA/B binding site to inward facing. The regulator has specificity for the clockwise pose by bridging elements unique to this conformation. We used these structures to propose how the switch reverses rotation and transmits torque to the flagellum, which advances the understanding of bacterial chemotaxis and bidirectional motor rotation.
Bacterial chemotaxis requires bidirectional flagellar rotation at different rates. Rotation is driven by a flagellar motor, which is a supercomplex containing multiple rings. Architectural uncertainty regarding the cytoplasmic C-ring, or 'switch', limits our understanding of how the motor transmits torque and direction to the flagellar rod. Here we report cryogenic electron microscopy structures for Salmonella enterica serovar typhimurium inner membrane MS-ring and C-ring in a counterclockwise pose (4.0 Å) and isolated C-ring in a clockwise pose alone (4.6 Å) and bound to a regulator (5.9 Å). Conformational differences between rotational poses include a 180° shift in FliF/FliG domains that rotates the outward-facing MotA/B binding site to inward facing. The regulator has specificity for the clockwise pose by bridging elements unique to this conformation. We used these structures to propose how the switch reverses rotation and transmits torque to the flagellum, which advances the understanding of bacterial chemotaxis and bidirectional motor rotation.
