EMD-6320
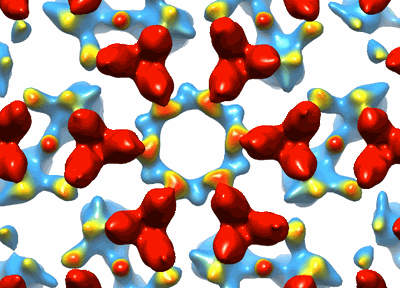
Structure of bacterial chemotaxis signaling CheA2-hexamer core complex by cryo-electron tomography and subvolume averaging
EMD-6320
Subtomogram averaging17.5 Å
 Deposition: 17/04/2015
Deposition: 17/04/2015Map released: 09/12/2015
Last modified: 09/12/2015
Sample Organism:
Escherichia coli
Sample: tarCF CheA CheW
Deposition Authors: Cassidy CK ,
Himes BA
,
Himes BA  ,
Alvarez FJ
,
Alvarez FJ  ,
Ma J,
Zhou G,
Perilla JR,
Schulten K,
Zhang P
,
Ma J,
Zhou G,
Perilla JR,
Schulten K,
Zhang P
Sample: tarCF CheA CheW
Deposition Authors: Cassidy CK
 ,
Himes BA
,
Himes BA  ,
Alvarez FJ
,
Alvarez FJ  ,
Ma J,
Zhou G,
Perilla JR,
Schulten K,
Zhang P
,
Ma J,
Zhou G,
Perilla JR,
Schulten K,
Zhang P
CryoEM and computer simulations reveal a novel kinase conformational switch in bacterial chemotaxis signaling.
Cassidy CK  ,
Himes BA
,
Himes BA  ,
Alvarez FJ
,
Alvarez FJ  ,
Ma J,
Zhou G,
Perilla JR,
Schulten K,
Zhang P
,
Ma J,
Zhou G,
Perilla JR,
Schulten K,
Zhang P
(2015) eLife , 4 , e08419 - e08419
 ,
Himes BA
,
Himes BA  ,
Alvarez FJ
,
Alvarez FJ  ,
Ma J,
Zhou G,
Perilla JR,
Schulten K,
Zhang P
,
Ma J,
Zhou G,
Perilla JR,
Schulten K,
Zhang P
(2015) eLife , 4 , e08419 - e08419
Abstract:
Chemotactic responses in bacteria require large, highly ordered arrays of sensory proteins to mediate the signal transduction that ultimately controls cell motility. A mechanistic understanding of the molecular events underlying signaling, however, has been hampered by the lack of a high-resolution structural description of the extended array. Here, we report a novel reconstitution of the array, involving the receptor signaling domain, histidine kinase CheA, and adaptor protein CheW, as well as a density map of the core-signaling unit at 11.3 Å resolution, obtained by cryo-electron tomography and sub-tomogram averaging. Extracting key structural constraints from our density map, we computationally construct and refine an atomic model of the core array structure, exposing novel interfaces between the component proteins. Using all-atom molecular dynamics simulations, we further reveal a distinctive conformational change in CheA. Mutagenesis and chemical cross-linking experiments confirm the importance of the conformational dynamics of CheA for chemotactic function.
Chemotactic responses in bacteria require large, highly ordered arrays of sensory proteins to mediate the signal transduction that ultimately controls cell motility. A mechanistic understanding of the molecular events underlying signaling, however, has been hampered by the lack of a high-resolution structural description of the extended array. Here, we report a novel reconstitution of the array, involving the receptor signaling domain, histidine kinase CheA, and adaptor protein CheW, as well as a density map of the core-signaling unit at 11.3 Å resolution, obtained by cryo-electron tomography and sub-tomogram averaging. Extracting key structural constraints from our density map, we computationally construct and refine an atomic model of the core array structure, exposing novel interfaces between the component proteins. Using all-atom molecular dynamics simulations, we further reveal a distinctive conformational change in CheA. Mutagenesis and chemical cross-linking experiments confirm the importance of the conformational dynamics of CheA for chemotactic function.
