EMD-6980
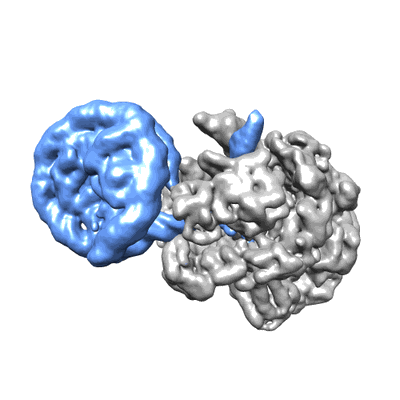
RNA polymerase II elongation complex stalled at SHL(-1) of the nucleosome, with foreign DNA
EMD-6980
Single-particle5.6 Å
 Deposition: 24/06/2018
Deposition: 24/06/2018Map released: 03/10/2018
Last modified: 27/03/2024
Sample Organism:
Komagataella phaffii (strain GS115 / ATCC 20864),
synthetic construct,
Homo sapiens
Sample: RNA polymerase II elongation complex stalled at SHL(-1) of the nucleosome, with foreign DNA
Fitted models: 6a5l (Avg. Q-score: 0.182)
Deposition Authors: Kujirai T ,
Ehara H
,
Ehara H  ,
Fujino Y,
Shirouzu M
,
Fujino Y,
Shirouzu M  ,
Sekine S,
Kurumizaka H
,
Sekine S,
Kurumizaka H 
Sample: RNA polymerase II elongation complex stalled at SHL(-1) of the nucleosome, with foreign DNA
Fitted models: 6a5l (Avg. Q-score: 0.182)
Deposition Authors: Kujirai T
 ,
Ehara H
,
Ehara H  ,
Fujino Y,
Shirouzu M
,
Fujino Y,
Shirouzu M  ,
Sekine S,
Kurumizaka H
,
Sekine S,
Kurumizaka H 
Structural basis of the nucleosome transition during RNA polymerase II passage.
Kujirai T  ,
Ehara H
,
Ehara H  ,
Fujino Y,
Shirouzu M
,
Fujino Y,
Shirouzu M  ,
Sekine SI
,
Sekine SI  ,
Kurumizaka H
,
Kurumizaka H 
(2018) Science , 362 , 595 - 598
 ,
Ehara H
,
Ehara H  ,
Fujino Y,
Shirouzu M
,
Fujino Y,
Shirouzu M  ,
Sekine SI
,
Sekine SI  ,
Kurumizaka H
,
Kurumizaka H 
(2018) Science , 362 , 595 - 598
Abstract:
Genomic DNA forms chromatin, in which the nucleosome is the repeating unit. The mechanism by which RNA polymerase II (RNAPII) transcribes the nucleosomal DNA remains unclear. Here we report the cryo-electron microscopy structures of RNAPII-nucleosome complexes in which RNAPII pauses at the superhelical locations SHL(-6), SHL(-5), SHL(-2), and SHL(-1) of the nucleosome. RNAPII pauses at the major histone-DNA contact sites, and the nucleosome interactions with the RNAPII subunits stabilize the pause. These structures reveal snapshots of nucleosomal transcription, in which RNAPII gradually tears DNA from the histone surface while preserving the histone octamer. The nucleosomes in the SHL(-1) complexes are bound to a "foreign" DNA segment, which might explain the histone transfer mechanism. These results provide the foundations for understanding chromatin transcription and epigenetic regulation.
Genomic DNA forms chromatin, in which the nucleosome is the repeating unit. The mechanism by which RNA polymerase II (RNAPII) transcribes the nucleosomal DNA remains unclear. Here we report the cryo-electron microscopy structures of RNAPII-nucleosome complexes in which RNAPII pauses at the superhelical locations SHL(-6), SHL(-5), SHL(-2), and SHL(-1) of the nucleosome. RNAPII pauses at the major histone-DNA contact sites, and the nucleosome interactions with the RNAPII subunits stabilize the pause. These structures reveal snapshots of nucleosomal transcription, in which RNAPII gradually tears DNA from the histone surface while preserving the histone octamer. The nucleosomes in the SHL(-1) complexes are bound to a "foreign" DNA segment, which might explain the histone transfer mechanism. These results provide the foundations for understanding chromatin transcription and epigenetic regulation.
