EMD-8285
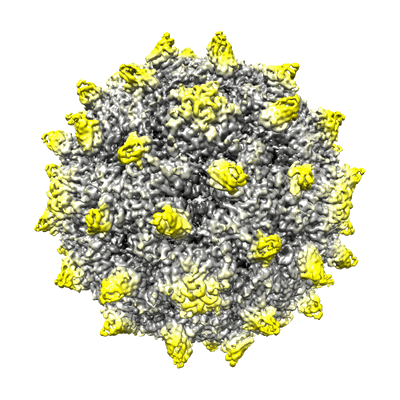
expanded poliovirus in complex with VHH 17B
EMD-8285
Single-particle5.3 Å
 Deposition: 23/07/2016
Deposition: 23/07/2016Map released: 02/11/2016
Last modified: 13/11/2024
Sample Organism:
Poliovirus type 1 (strain Mahoney),
Camelus dromedarius
Sample: expanded poliovirus in complex with VHH 17B
Fitted models: 5ku0 (Avg. Q-score: 0.348)
Deposition Authors: Strauss M, Schotte L, Filman DJ ,
Hogle JM
,
Hogle JM
Sample: expanded poliovirus in complex with VHH 17B
Fitted models: 5ku0 (Avg. Q-score: 0.348)
Deposition Authors: Strauss M, Schotte L, Filman DJ
 ,
Hogle JM
,
Hogle JM
Cryo-electron Microscopy Structures of Expanded Poliovirus with VHHs Sample the Conformational Repertoire of the Expanded State.
Abstract:
By using cryo-electron microscopy, expanded 80S-like poliovirus virions (poliovirions) were visualized in complexes with four 80S-specific camelid VHHs (Nanobodies). In all four complexes, the VHHs bind to a site on the top surface of the capsid protein VP3, which is hidden in the native virus. Interestingly, although the four VHHs bind to the same site, the structures of the expanded virus differ in detail in each complex, suggesting that each of the Nanobodies has sampled a range of low-energy structures available to the expanded virion. By stabilizing unique structures of expanded virions, VHH binding permitted a more detailed view of the virus structure than was previously possible, leading to a better understanding of the expansion process that is a critical step in infection. It is now clear which polypeptide chains become disordered and which become rearranged. The higher resolution of these structures also revealed well-ordered conformations for the EF loop of VP2, the GH loop of VP3, and the N-terminal extensions of VP1 and VP2, which, in retrospect, were present in lower-resolution structures but not recognized. These structural observations help to explain preexisting mutational data and provide insights into several other stages of the poliovirus life cycle, including the mechanism of receptor-triggered virus expansion.
By using cryo-electron microscopy, expanded 80S-like poliovirus virions (poliovirions) were visualized in complexes with four 80S-specific camelid VHHs (Nanobodies). In all four complexes, the VHHs bind to a site on the top surface of the capsid protein VP3, which is hidden in the native virus. Interestingly, although the four VHHs bind to the same site, the structures of the expanded virus differ in detail in each complex, suggesting that each of the Nanobodies has sampled a range of low-energy structures available to the expanded virion. By stabilizing unique structures of expanded virions, VHH binding permitted a more detailed view of the virus structure than was previously possible, leading to a better understanding of the expansion process that is a critical step in infection. It is now clear which polypeptide chains become disordered and which become rearranged. The higher resolution of these structures also revealed well-ordered conformations for the EF loop of VP2, the GH loop of VP3, and the N-terminal extensions of VP1 and VP2, which, in retrospect, were present in lower-resolution structures but not recognized. These structural observations help to explain preexisting mutational data and provide insights into several other stages of the poliovirus life cycle, including the mechanism of receptor-triggered virus expansion.
