EMD-27692
LM18/Nb136 bispecific tetra-nanobody immunoglobulin in complex with SARS-CoV-2-6P-Mut7 S protein (focused refinement)
EMD-27692
Single-particle3.34 Å
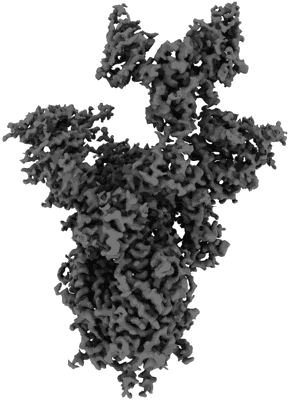 Deposition: 25/07/2022
Deposition: 25/07/2022Map released: 14/06/2023
Last modified: 20/11/2024
Sample Organism:
Severe acute respiratory syndrome coronavirus 2,
synthetic construct
Sample: LM18/Nb136 bispecific tetra-nanobody immunoglobulin in complex with SARS-CoV-2-6P-Mut7 S protein
Fitted models: 8dt8 (Avg. Q-score: 0.443)
Deposition Authors: Ozorowski G ,
Turner HL,
Ward AB
,
Turner HL,
Ward AB 
Sample: LM18/Nb136 bispecific tetra-nanobody immunoglobulin in complex with SARS-CoV-2-6P-Mut7 S protein
Fitted models: 8dt8 (Avg. Q-score: 0.443)
Deposition Authors: Ozorowski G
 ,
Turner HL,
Ward AB
,
Turner HL,
Ward AB 
Fully synthetic platform to rapidly generate tetravalent bispecific nanobody-based immunoglobulins.
Misson Mindrebo L  ,
Liu H
,
Liu H  ,
Ozorowski G
,
Ozorowski G  ,
Tran Q
,
Tran Q  ,
Woehl J
,
Woehl J  ,
Khalek I,
Smith JM
,
Khalek I,
Smith JM  ,
Barman S,
Zhao F
,
Barman S,
Zhao F  ,
Keating C,
Limbo O
,
Keating C,
Limbo O  ,
Verma M
,
Verma M  ,
Liu J
,
Liu J  ,
Stanfield RL
,
Stanfield RL  ,
Zhu X
,
Zhu X  ,
Turner HL,
Sok D,
Huang PS
,
Turner HL,
Sok D,
Huang PS  ,
Burton DR
,
Burton DR  ,
Ward AB
,
Ward AB  ,
Wilson IA
,
Wilson IA  ,
Jardine JG
,
Jardine JG 
(2023) PNAS , 120 , e2216612120 - e2216612120
 ,
Liu H
,
Liu H  ,
Ozorowski G
,
Ozorowski G  ,
Tran Q
,
Tran Q  ,
Woehl J
,
Woehl J  ,
Khalek I,
Smith JM
,
Khalek I,
Smith JM  ,
Barman S,
Zhao F
,
Barman S,
Zhao F  ,
Keating C,
Limbo O
,
Keating C,
Limbo O  ,
Verma M
,
Verma M  ,
Liu J
,
Liu J  ,
Stanfield RL
,
Stanfield RL  ,
Zhu X
,
Zhu X  ,
Turner HL,
Sok D,
Huang PS
,
Turner HL,
Sok D,
Huang PS  ,
Burton DR
,
Burton DR  ,
Ward AB
,
Ward AB  ,
Wilson IA
,
Wilson IA  ,
Jardine JG
,
Jardine JG 
(2023) PNAS , 120 , e2216612120 - e2216612120
Abstract:
Nanobodies bind a target antigen with a kinetic profile similar to a conventional antibody, but exist as a single heavy chain domain that can be readily multimerized to engage antigen via multiple interactions. Presently, most nanobodies are produced by immunizing camelids; however, platforms for animal-free production are growing in popularity. Here, we describe the development of a fully synthetic nanobody library based on an engineered human VH3-23 variable gene and a multispecific antibody-like format designed for biparatopic target engagement. To validate our library, we selected nanobodies against the SARS-CoV-2 receptor-binding domain and employed an on-yeast epitope binning strategy to rapidly map the specificities of the selected nanobodies. We then generated antibody-like molecules by replacing the VH and VL domains of a conventional antibody with two different nanobodies, designed as a molecular clamp to engage the receptor-binding domain biparatopically. The resulting bispecific tetra-nanobody immunoglobulins neutralized diverse SARS-CoV-2 variants with potencies similar to antibodies isolated from convalescent donors. Subsequent biochemical analyses confirmed the accuracy of the on-yeast epitope binning and structures of both individual nanobodies, and a tetra-nanobody immunoglobulin revealed that the intended mode of interaction had been achieved. This overall workflow is applicable to nearly any protein target and provides a blueprint for a modular workflow for the development of multispecific molecules.
Nanobodies bind a target antigen with a kinetic profile similar to a conventional antibody, but exist as a single heavy chain domain that can be readily multimerized to engage antigen via multiple interactions. Presently, most nanobodies are produced by immunizing camelids; however, platforms for animal-free production are growing in popularity. Here, we describe the development of a fully synthetic nanobody library based on an engineered human VH3-23 variable gene and a multispecific antibody-like format designed for biparatopic target engagement. To validate our library, we selected nanobodies against the SARS-CoV-2 receptor-binding domain and employed an on-yeast epitope binning strategy to rapidly map the specificities of the selected nanobodies. We then generated antibody-like molecules by replacing the VH and VL domains of a conventional antibody with two different nanobodies, designed as a molecular clamp to engage the receptor-binding domain biparatopically. The resulting bispecific tetra-nanobody immunoglobulins neutralized diverse SARS-CoV-2 variants with potencies similar to antibodies isolated from convalescent donors. Subsequent biochemical analyses confirmed the accuracy of the on-yeast epitope binning and structures of both individual nanobodies, and a tetra-nanobody immunoglobulin revealed that the intended mode of interaction had been achieved. This overall workflow is applicable to nearly any protein target and provides a blueprint for a modular workflow for the development of multispecific molecules.
