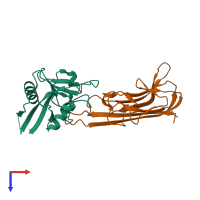
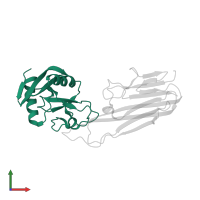
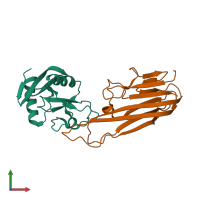Assemblies
Assembly Name:
Tetranectin and Tumor necrosis factor, soluble form
Multimeric state:
hetero dimer
Accessible surface area:
13764.58 Å2
Buried surface area:
1791.96 Å2
Dissociation area:
844.97
Å2
Dissociation energy (ΔGdiss):
-4.61
kcal/mol
Dissociation entropy (TΔSdiss):
11.35
kcal/mol
Symmetry number:
1
PDBe Complex ID:
PDB-CPX-134275
Macromolecules
Chain: C
Length: 134 amino acids
Theoretical weight: 15.31 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
Pfam: Lectin C-type domain
InterPro:
CATH: Mannose-Binding Protein A, subunit A
Length: 134 amino acids
Theoretical weight: 15.31 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
- Canonical:
 P05452 (Residues: 67-201; Coverage: 74%)
P05452 (Residues: 67-201; Coverage: 74%)
Pfam: Lectin C-type domain
InterPro:
CATH: Mannose-Binding Protein A, subunit A
Chain: T
Length: 149 amino acids
Theoretical weight: 16.5 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
Pfam: TNF(Tumour Necrosis Factor) family
InterPro:
Length: 149 amino acids
Theoretical weight: 16.5 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
- Canonical:
 P01375 (Residues: 85-233; Coverage: 64%)
P01375 (Residues: 85-233; Coverage: 64%)
Pfam: TNF(Tumour Necrosis Factor) family
InterPro:
- Tumour necrosis factor-like domain superfamily
- Tumour necrosis factor
- Tumour necrosis factor domain
- Tumour necrosis factor alpha
- Tumour necrosis factor, conserved site